Introduction
Following the United Kingdom – United States Cancer Summit, the Medical Research Council (MRC – UKRI) and the National Institute for Health and Care Research (NIHR), in partnership with the National Institutes of Health (NIH) National Cancer Institute (NCI), are launching a second competition for the Cancer Research Transatlantic Development and Skills Enhancement (CRT-DSE) award.
This award, which is jointly funded by the MRC – UKRI and the NIHR, with in-kind support from the NIH NCI, will provide a unique opportunity for post-doctoral UK early/mid career researchers to spend between 6-12 months at the world-class NCI Intramural Institutes, Centre for Cancer Research or Division of Cancer Epidemiology and Genetics, in Bethesda, United States (US).
Successful applicants will be supported to undertake training, develop skills and experience, and establish new (or substantially develop existing) international collaborations.
Who is the award for?
The CRT-DSE is for UK-based early/mid-career researchers who are looking to gain training, develop skills and experience, and establish international collaborations with NCI researchers. It will support a transition to independence in cancer research.
All applicants must propose a clear and detailed training and development plan that will maximise the opportunity provided through the scheme to work with US collaborators, with support of their host institution and mentors. The CRT-DSE can support individuals at a broad range of post-doctoral career stages (including MRCs "Early Post-doctoral and Transition to independence" career stages) however all applicants must clearly demonstrate that they have the potential, and are on a trajectory, to become future leaders in their area of research and describe how this award will support them to achieve this. Early post-doctoral applicants particularly will need to provide evidence of productivity and potential to lead independent research, for example as demonstrated by critical contributions to independent or collaborative research outputs and articulate how this award will support a step change in their career trajectory towards independence and leadership.
Whilst not ineligible, individuals that are already established as an independent researcher, are not the priority career stage targeted for this scheme. If an established researcher applies, a clear justification should be provided to explain why the support is required and why it is not already available through the Host Organisation. Applicants should consider whether this award will provide significant support on their career trajectory.
The CRT-DSE supports UK-based post-doctoral clinical or non-clinical researchers who are working in any areas of cancer research within MRC-UKRI and/ or NIHR’s remit across biomedical and health research (for example discovery and basic disease biology, through to translational, clinical and applied cancer research).
We are particularly keen to see applications that develop skills and experience in the following areas that align with priority opportunities identified during the UK-US Cancer Summit:
- Data science and AI
- Health equity
- Molecular and other mechanisms of cancer prevention
- Trial methodology including innovative clinical trial design and delivery
Applicants are likely to have a background in cancer research. Applicants that do not come from a cancer research background are eligible but will be expected to demonstrate how the opportunity would facilitate ongoing cancer research and sustained collaborations beyond the end of the award.
Eligibility Criteria
To be eligible, applications must meet the following criteria:
- Applicants must hold a relevant PhD or MD at the time of awarding.
- Individuals must not already hold a Chair at the point of awarding.
- Applicants must have the potential, and be on a trajectory, to become future leaders in cancer research within MRC-UKRI and/ or NIHR’s remit
- The proposed Host Organisation (the organisation who will be the contractor if the award is funded and the applicant’s substantive employer) must be an HEI, NHS body, other provider of health and/or care services located in the UK or other organisation eligible for UKRI funding.
- For clinical academic applications: applicants must have completed the relevant pre-registration training.
- The proposed US placement (the NCI Host) must be at one of the two intramural centres directly funded by the NCI, Centre for Cancer Research or Division of Cancer Epidemiology and Genetics, in Bethesda US (see “NCI Host” below).
Applications in which the applicant is living overseas from their host UK organisation will not be supported. Cancer Research Transatlantic DSE award holders must be based in the UK at their host organisation for the duration of the award, except when visiting the NCI Host.
Applicants do not need to currently hold an MRC – UKRI or NIHR award/fellowship, but current award holders are eligible to apply.
Applicants are encouraged to contact NIHR and MRC-UKRI if they have any queries regarding their eligibility or scope of the scheme. For queries please contact:
Email: academy-awards@nihr.ac.uk
Tel: 0113 532 8410
Training and Development Programme and Development of Collaborations
This award will provide funding and support for award holders to spend between 6 to 12 months at NCI-intramural institutes in the United States. More information on eligible research groups is provided below.
Applicants can request awards of between 6-12 months and the format of the visits is flexible. For example, applicants may choose to make one visit for an extended duration or make several shorter visits. MRC – UKRI and NIHR are supportive of applicants wishing to combine this award with caring / family responsibilities, and appropriate costs to support this can be included within the application (see “Funding and Support Available”).
Applicants must spend substantial time within the duration of the award visiting one of the world-class NCI Intramural Institutes, Centre for Cancer Research or Division of Cancer Epidemiology and Genetics, in Bethesda US (NCI Host) and the time spent overseas must accommodate the specific training and skills development aims of each application and the applicants’ individual circumstances. Whilst virtual interactions will support ongoing development and skills over the duration of the award, taking advantage of the opportunity for in-person visits is expected to be prioritised.
The proposed time with the NCI Host group should support the applicant’s transition to independence in cancer research, their development of international collaborations and their gaining of skills and experience to underpin the next phase of their cancer research career.
Co-mentoring will be expected in both the UK and US, to promote cross-partner working and ensure effective career development support. It is hoped successful applicants will share their learning and experience with colleagues and other researchers on their return to the UK.
Competitive applications will include full details of the activities that will be achieved through the time spent with the NCI group and how it will benefit their future career in cancer research.
In your application, you must clearly justify:
- How the proposed activities align with your existing skills and experiences
- What training, skills and experiences will be gained through the award including new/developed collaborations
- How the proposed activities will support and enable skills development and knowledge transfer
- The choice of NCI research group proposed for a partnership and how this collaboration will support and enable your long-term career goals in cancer research
- What mentoring and support will be in place to ensure maximum value from this opportunity
- The proportion and pattern of time spent overseas and in the UK
- How this award will support and enable your long-term career goals in cancer research
Activities undertaken through a Cancer Research Transatlantic DSE may include (but are not limited to):
- Development of research skills (e.g. experimental techniques, data analytics, trial methodologies) and research activities (e.g., continuation of the applicant’s existing research, collecting pilot data or conducting preliminary work to support future funding applications)
- Expanding networks and forming new collaborations (which may include industry, charity or patient advocacy partners)
- Formal and informal training courses. Applicants can also consider wider development training (eg. Leadership)
- Placements spending time in clinical research settings (e.g. training within clinical trials units, hospitals, medical research centres)
- Training in or development of skills to undertake Patient and Public Involvement
- Conference attendance
- Any other development of skills or experience which would benefit future applications and longer-term career plans
Competitive applicants will develop a well-rounded programme of activities that take full advantage of the opportunities available at the NCI Host.
Funding and Support available
A Cancer Research Transatlantic DSE award provides funding for:
- The salary of the award holder for 6-12 months
- Travel, accommodation and subsistence costs associated with visitstotheNCI Host in the United States
- Includes both personal and family relocation costs
- Includes visa costs and health insurance
- Subsistence costs to reflect the increase in cost of living can be requested. The NIHR reserves the right to review and adjust these costs to ensure fair and justified support for all applicants.
- Training and development costs up to £5,000
- Conference costs up to £2,000
- Research costs and consumables
- NCI partner research groups will support standard consumables, but where substantial or exceptional additional research costs are required for the proposed research and skills development, costs may be requested where justified.
- Funding for clinical trials can not be requested, however, costs for collecting pilot data and conducting preliminary work to support future research funding applications can be included.
All costs will be paid at 100%. Full Economic Costings (FEC) are not covered by this award.
Identifying and Contacting NCI Hosts
Eligible research groups for the Cancer Research Transatlantic DSE are directly funded by the NCI at the Center for Cancer Research (CCR) or the Division of Cancer Epidemiology and Genetics (DCEG). For more information about eligible NCI research groups, please see:
- Research areas at NCI’s Center for Cancer Research (CCR)
- Fields of study at NCI’s Division of Cancer Epidemiology and Genetics (DCEG)
Applicants with existing contacts, or those who are clear on who they wish to approach, are welcome to approach potential NCI hosts directly. If prospective applicants are unsure which research groups may be best suited to their interests, support and “match making” can be facilitated by contacting NCI central contacts. An email inquiry should be sent to both Erika Ginsburg and Jackie Lavigne and include the following information:
- Up to 3 sentences describing your scientific focus area(s)
- CV
Applicants requiring this support should get in touch as early as possible and no later than 7th June 2024).
Once matching with a research group has been undertaken by NCI, the prospective applicant will be informed via email of the proposed research group and provided with their contact details.
All applicants are required to work with their prospective NCI Host to develop their application, discussing the opportunities and activities that will be on offer. As part of the application, the NCI Host is required to provide a letter of support, which the applicant will upload as part of their application (see “Statement of Support” below).
VISAs
The NCI Host will arrange VISAs for successful applicants. To progress a VISA application, the NCI administrators will require the following documents 4 months in advance of the proposed start date:
- CV and Bibliography
- Three letters of reference – letters must be dated within the last year and signed
- Copy of degree and/or degree certification/school verification (see information about foreign education verification and translation)
- Visiting Fellow Agreement
- Div of International Services (DIS) forms
Applicants can consider starting a placement virtually from the UK before relocation to the US where suitable.
Health Insurance
Successful applicants will be responsible for ensuring that they have the required Health Insurance coverage to enable them to visit the NCI Host. Those who fail to obtain adequate health insurance coverage will be unable to access NIH facilities. Costs for Health Insurance for both the award holder and any dependents travelling with them can be requested through the award. For full details of the requirements, please see NIH Policy Manual on Special Volunteers.
Host Organisation
Applicants need to identify an eligible UK Host Organisation which will act as their employer for the duration of the award and the contractor if the award is funded.
Any organisation wishing to host this award must be able to provide the applicant with a contract of employment for the duration of the award and be capable of fulfilling the role of research sponsor as set out in the Research Governance Framework for Health & Care. Further guidance on the roles and responsibilities of a research sponsor can be found on the Health Research Authority’s (HRA) website.
Host Organisations can either be a recognised Higher Education Institute (HEI) or any other organisation which provides health or social care services and is based in the UK and in receipt of public funding (for example, social enterprises or local authorities) or other organisations eligible for UKRI funding.
This award requires that the award holder has a contract of employment with the Host Organisation for the duration of the Award. The Department of Health and Social Care (DHSC) will enter into a contract with the Host Organisation. Government procurement transparency regulations require the publication of all contracts made with the DHSC to be made available on the DHSC website. Confidential information including research proposals, detailed finance information, bank details, and departmental staff names (other than the award holder’s name) will be removed from the published versions.
Statements of Support
Both the UK host (applicant employer and contractor if the application is successful) and NCI Host (organisation being visited) are required to provide applicant-specific statements of support that outline appropriate commitment to supporting skills development, training needs and long-term career goals.
UK Host Organisation Commitment (Head of Department completes a “Statement of Support” question on the application form)
The UK Host Organisation Statement of support should include the following information:
- The support that will be provided by the UK Host Organisation to enable development and progression of the applicant’s research career.
- How the UK Host Organisation will liaise with the NCI Host Organisation to ensure appropriate support is in place, including arrangements for pastoral care.
- The names of senior academic(s) who have supported the applicant during the development of the application and who will continue to do so over the course of the award.
- Evidence of the Host organisation’s commitment to creating and maintaining an inclusive and supportive research culture, including evidence of commitment to the principles of equality, diversity and inclusion, and research integrity.
NCI Host Commitment (Collaboration Letter uploaded to the application form)
The NCI Host Collaboration Letter should include the following information:
- Additional details of in-kind commitment including access to space or equipment
- Support that will be provided by the NCI host to enable development and progression of the applicant’s research career
- How the US host organisation will liaise with the UK host organisation to ensure appropriate support is in place, including arrangements for pastoral care.
- The names of senior academics who have supported the applicant during the development of the application and who will continue to do so, including any planned mentorship arrangements.
- Evidence of the US host organisation’s commitment to creating and maintaining an inclusive and supportive research culture, including evidence of commitment to the principles of equality, diversity and inclusion, and research integrity.
The statements of support from both the UK host and US host are reviewed by the Committee as part of the assessment criteria.
Ethical considerations
Applicants must ensure that all proposed research, both that in the UK and in the US, will comply with the standard principles and expectations for UK research and MRC – UKRI and NIHR policies.
Additional UKRI Requirements
From September 2022, MRC – UKRI will require both sexes to be used in the experimental design of grant applications involving animals, and human and animal tissues and cells, unless there is a strong justification for not doing so.
Joint letters of support accompanying the application will be required when human participation / human tissue or animal research is proposed. Letters should state that all applicants will comply with the relevant MRC – UKRI policies and guidance and must be signed by both the applicant and the NCI host. For more information see ‘Additional Supporting Information’ Section.
Application deadlines and timetable
Applications must have been submitted by the deadline and the signatory must have approved the application by this time using the Academy Research Awards Management Information System (ARAMIS) online application system. All components of your application, including supporting documents for the Uploads section, must be submitted by the deadline.
Timetable
Competition opens for applications: 2nd April 2024
Webinar for prospective applicants: 25th April 2024, 2 - 3pm Register on Zoom
Deadline for registering interest for match-making with NCI (if required): 7th June 2024 (12pm)
Application submission deadline: 2nd August 2024 by 1pm
Awards start on 1st of month: 1 April 2025 to 1 July 2025
Assessment Criteria
Cancer Research Transatlantic DSE applicants will be expected to demonstrate a continued commitment to a cancer research career in an area of relevance to NIHR and/or MRC – UKRI and a clearly articulated plan for how the award will support their career in cancer research including the skills and experience they will gain by undertaking the award.
The Cancer Research Transatlantic DSE Selection Committee will assess each application against the following criteria;
Applicant
- Relevant previous experience
- Continued commitment to a cancer research career in an area of relevance to NIHR and/or MRC – UKRI
- Clear articulation of future research career plans and how the award will support them to reach the next stage of their cancer research career
- Evidence of productivity and potential to lead independent research, (for example as demonstrated by critical contributions to relevant independent or collaborative research outputs) and an upwards trajectory to become a future biomedical, health or social care research leader
Training, Development and Collaborations
- Clear articulation of the skills and experience that the applicant will gain by undertaking the award.
- Clear articulation of how the proposed placement will enhance the applicant’s cancer research career and enable transatlantic cancer research collaborations in the future
- Clear articulation of the proposed research activity that will be undertaken and how this will be enabled by the skills and experience that the applicant will gain in collaboration with NCI hosts.
- Suitability of the proposed placement - does the researcher's area of expertise and proposed research/training ambitions align with potential collaborators in the US, indicating a strong basis for productive collaborations?
- Evidence of how this Development and Skills Enhancement award can be managed alongside existing awards and commitments.
Support
- Host Organisation: Appropriateness and quality of the proposed support and mentorship.
- NCI Host: Appropriateness and quality of the proposed support and mentorship
- Evidence of the host organisation(s) and Host NCIs commitment to creating and maintaining an inclusive and supportive research culture, including evidence of commitment to the principles of equality, diversity and inclusion and research integrity.
- Host Organisation has a demonstrable track record in training and supporting people who have gone on to develop successful research careers.
CRT-DSE Deliverables
Upon completion of a CRT-DSE, awardees should be able to demonstrate the following:
- Completion of the Training and Development Programme:
- Fulfil all requirements outlined in the submitted application, ensuring comprehensive participation in the training and development programme.
- Enhanced Independence as a Researcher:
- Showcase increased autonomy and self-reliance in research endeavours.
- Provide tangible examples of how the training has contributed to a heightened ability to design, conduct, and analyse research independently.
- Promotion of Collaborative Initiatives:
- Actively engage in collaborations with peers, mentors, and relevant stakeholders as a result of the acquired skills and knowledge.
- Highlight collaborative projects, publications, or other outputs that demonstrate a commitment to knowledge-sharing and interdisciplinary cooperation.
- Trajectory Towards Leadership:
- Demonstrate a clear and significant advancement in the trajectory toward becoming a leader in the field of cancer research.
- Showcase achievements, contributions, or initiatives that signify a step-change in the applicant's professional development and leadership potential.
Monitoring and Reporting:
NIHR will assess the progress of awards through regular monitoring, including both annual and final reports submitted by award recipients.
Application Procedure and Selection Process
- NIHR Academy uses an adapted version of the NIHR Standard Application Form (SAF), which is a 1 stage process.
- NIHR Academy can advise on eligibility and remit enquiries, and answer queries you may have when completing the application form.
- All applications will undergo an initial screen for eligibility. Following this, applications are reviewed by the Selection Committee in advance of a funding recommendation meeting at which the committee discuss the applications and make recommendations for funding to the Department of Health and Social Care (DHSC).
- Details of Selection Committee membership will be published on the NIHR website.
- The final decision as to which applications will receive funding rests with DHSC and MRC – UKRI with final decision resting with DHSC as the awarding agency. Once confirmed, the funding decisions will be communicated to applicants.
- Applicants will be informed of the outcome of their application by email when all required processes are complete.
- The selection process and subsequent management of the Awards will be managed by NIHR.
- All documents must be submitted in English.
- Awards cannot be deferred, without the consent of the NIHR.
Only one application to the Cancer Research Transatlantic DSE is permitted per round. Multiple applications will not be accepted.
Guidance on completing the application form
Registering
All applications must be completed and submitted via ARAMIS, the NIHR online application system.
Before you can start an application you will be required to register on the system. You will be asked to supply a valid email address and to complete some basic information. Once this has been submitted you will receive an email confirming your registration and a temporary password. You should follow the instructions in the email to log onto the system.
Once signed into the system you will be able to update various details including your CV (in ‘manage my details’) and apply for any open applications. To start an application you will need to go to ‘My Applications’ and select ‘New Application’. You should then select the Cancer Research Transatlantic Development and Skills Enhancement Award from the list provided.
After answering all the eligibility questions you will be able to start completing the online form. Please make sure you read all available guidance text including this document as well as any online instructions thoroughly whilst you are completing the form. You can at any stage download a PDF version of the application which can be useful for sharing applications with others.
1. Application Summary Information
Host Organisation
Please give details of the UK organisation who will be the contractor if the award is funded.
Please note that we expect the applicant’s proposed host organisation (substantive employer) to act as the contractor.
Please also bear in mind that:
The contractor is expected to respond to annual financial reconciliation exercises, provide the final financial reconciliation statement for the award and to provide ad hoc requests for financial information during the lifetime of the award.
In the same way, the contractor is expected to respond to any queries relating to Intellectual Property, commercialisation and benefit realisation.
If the name of your host organisation does not appear in the pre-populated list please email academy-awards@nihr.ac.uk.
Proposed Start Date
Note this should be from 1st of the month regardless of whether this is a working day or not. Please be realistic about your possible start date taking account of the necessary contracting, and staff recruitment prior to starting your project.
WTE of Award
Cancer Research Transatlantic DSE awards are a minimum 6 months and maximum 12 months in duration. You can choose to undertake the award between 50 and 100% WTE.
2. Applicant CV
Please note some of the responses to these questions are automatically pulled through from information you have entered in the ’Manage My Details’ page.
The ‘Publication Record’ section of the form is automatically populated from publications added into the “My Research Outputs” page of your ARAMIS account.
Degrees and Professional Qualifications
Please give the full details of any key qualifications completed and, where relevant, the full details of any key qualifications you are currently undertaking.
This information can be edited in the ‘Manage My Details’ page of your ARAMIS account.
Present and previous positions
Please give the full details of any relevant present and previous positions.
This information can be edited in the ‘Manage My Details’ page of your ARAMIS account.
When entering details of your current and previous positions please indicate at what percentage (WTE) in each post you were undertaking research. For example, if you were a Clinical Lecturer and undertook research for 2.5 days a week and clinical work for 2.5 days per week; please enter 50% for that position. If you have worked part time at 60%, and undertook research for half of that time, please enter 30% for that position.
Research grants held
Please give the details of all relevant grants obtained in the last five years, including personal research training awards or fellowships, plus any additional previous grants relevant to this application. Please indicate clearly any co-applicants and provide brief details of the nature and full extent of your involvement (e.g. project design, project management, day to day running, data collection, data analysis, writing papers for publication, etc.).
Please also include in the ‘Role in Research Grant’ box for each entry: registration number and name of registry and the DOI of the main related publication. Where the study is still ongoing or final results have not yet been published, please provide an estimated publication date. This is in line with the NIHR policy on clinical trial registration and disclosure of results.
Please note that your research grant record must be completed within the application form and not via the CV section on ARAMIS.
Publication Record
The publication record is automatically populated from the information added to the “Research Outputs” section of your ARAMIS account. To update, please “save and close” your application, return to the “home” screen and select “Research Outputs” from the left-hand menu.
When publications have been added, the Lead Applicant name can be edited to show in bold within application forms via the “Assign Grant Contacts” option.
To ensure publications display correctly, with all of the required information, applicants are strongly advised to use the “import” function and import their publications from Europe PMC.
Do not include abstracts, conference proceedings or articles in preparation. If relevant, details of these may be included in the ‘Applicant Research Background’ section. Details of articles which are in press and have been accepted as final by the publisher may be included. Depending on professional background and expertise, applicants are not necessarily expected to have an extensive list of publications.
Only publications relevant to your application should be included.
Relevant Prizes, Awards and other Academic Distinctions
Please provide details of any awards or distinctions that are relevant to your application including details of what the award was for. For example, travel bursaries for a conference, presentation prizes etc.
ORCiD
The NIHR is an ORCID member and encourages all researchers to obtain this persistent digital identifier that distinguishes you from every other researcher. You must include an ORCID ID in your application. Without it, your application will not be validated and you will not be able to submit. For more information and to register please visit the ORCHID website.
3. Applicant Research Background
Professional background
Select the one option which best describes your professional group. This will determine the options that appear below for your professional background.
Please describe your research career to date
Please use this question to provide a narrative describing your relevant research experience and career to date and how this makes you suitable for this award. All applicants should describe contributions across the four sections below
Contribution to knowledge
Explain how you have contributed to the generation of new ideas and hypotheses, and which key skills you have used and how you have communicated on your ideas to develop ideas and test hypotheses. You can highlight contributions and skills acquired from previous roles and research projects, funding you have won, awards received, and a small selection of key outputs explaining why they are of particular relevance and why they are considered in the context of knowledge generation. Where outputs have a DOI please only include this.
Contribution to the development of others
You might include experiences from project management, supervision, mentoring or line management contributions to the success of a team or team members, establishment of collaborations, or where you exerted strategic leadership in shaping the direction of a team, organisation, company or institution. Highlight expertise you provided which was critical to the success of a team or team members e.g., project management, strategic leadership, teaching activities, mentoring, and establishment of collaborations.
Contribution to the wider research community
Highlight activities where you have engaged in to progress the research community e.g., commitments such as committee memberships, editing and reviewing and contributions to the evaluation of researchers and research projects. You can also highlight contributions to increasing research integrity and improving research culture (gender equality, diversity, mobility of researchers, reward, and recognition of researchers’ various activities).
Contribution to broader society
Provide examples of where you have been involved in societal engagement and knowledge exchange i.e. engagement with patients and the public, industry, across private sector and public sectors clients and with the broader public. Describe where you have contributed to policy development or public understanding, and other impacts across research, policy, practice and business, and other examples of and how you have ensured research reaches and influences relevant audiences.
Has this application been previously submitted to this or any other funding body?
Select ‘Yes’ or ‘No’ to indicate whether this or a similar application has previously been submitted to this or any other funding body. This must include any previous submissions for an NIHR research training award, even if the proposed training programme has changed. Please detail the title of any previous submission(s), the funding body and scheme, the outcome and the date this is due if a decision is pending. If the application was unsuccessful please indicate why and detail how this application differs from previous submission(s) and how any feedback received has been used to inform this application.
Current and previous NIHR awards
In order to help track the progression of NIHR academy members please indicate whether you have previously held or currently hold another NIHR research training award. Please indicate in this section if you are applying as part of the NIHR Infrastructure; for example BRCs, ARCs etc.
Contextual factors
Please use this question to detail any contextual factors you wish to make the Selection Committee aware of. NIHR wants to know about any circumstances so that they may take them into consideration during the assessment of your application. Contextual factors may include:
- Career breaks due to parental leave, or periods of illness.
- Reduced time spent undertaking research due to a disability or caring responsibilities. This could include any physical or mental difficulty that may have impacted your research career. These are situations that have a significant impact on your ability to undertake research.
- Reduced opportunities to career support e.g. mentorship, and limited opportunities to undertake prior research and training.
- Impact of the COVID-19 pandemic on your research career.
Please also use this section to detail any other factors that may have impacted your research career not listed in the examples provided. The impact on your career to date will be specific to your particular circumstances but could include such impacts as limited opportunities to obtain grant funding, or fewer publications. In general terms, contextual factors should be significant, and relevant.
NIHR acknowledges that you may be reluctant, or uncomfortable disclosing relevant information that is sensitive. However, you should bear in mind that we are unable to take into account factors that you do not disclose. Please be assured that information provided by you is sensitive and will be treated confidentially and in line with General Data and Protection Regulations (GDPR).
4. Training and Development and Research Support
Proposed training and development programme and Collaborations
Please use this section to provide details of the training and skills development you will undertake as part of the award. This could include both training you may need to undertake to support future research plans but also training designed to support your development as a future biomedical, health and/or social care research leader.
Activities undertaken through a Cancer Research Transatlantic DSE may include (but are not limited to):
- Development of research skills (e.g., experimental techniques, data analytics, trial methodologies) and research activities (e.g., continuation of the applicant’s existing research, collecting pilot data or conducting preliminary work to support future funding applications)
- Expanding networks and forming new collaborations (which may include industry, charity or patient advocacy partners)
- Formal and informal training courses. Applicants can also consider wider development training (eg. Leadership)
- Placements spending time in clinical research settings (e.g. training within clinical trials units, hospitals, medical research centres)
- Training in or development of skills to undertake Patient and Public Involvement
- Conference attendance
- Any other development of skills or experience which would benefit future applications and longer-term career plans
Please ensure you also articulate why the activities described have been chosen and how these activities will enable your future cancer research career.
Please describe the impact this award will have on your future career, including details of why you are looking to undertake this award and where you see your career going as a result of doing so. Specifically, how will the proposed training and development enhance your chances of achieving your career goals.
Research support
Co-mentoring will be expected in both the UK and US, to promote cross-partner working and ensure effective career development support. Please detail here the UK-based individuals that will provide research support (mentorship), and the support that will be provided (including the reason for the choice).
The individuals you list here must also be added in the ‘Participants and Signatories’ section of the application form.
Although we acknowledge that formal supervision may not be appropriate for applicants, we believe that the applicant will benefit from research support (mentorship). The individuals who provide research support may or may not be based in your host organisation. They should, however, have a clear understanding of the research process, the demands your chosen area of training and development are likely to place on you, and your particular strengths and weaknesses.
Research support is referred to in the literature as ‘mentorship’ and there are numerous models to be found that could be employed. Clearly describe how the proposed arrangements will support your overall development and provide an initial assessment of the time that will be allocated to the research support process.
NCI Collaborations
Please use this section to detail both the collaborations that will be developed during the award and the US -based mentorship arrangements proposed.
Mentorship
Co-mentoring will be expected in both the UK and US, to promote cross-partner working and ensure effective career development support. Please detail here the US-based individuals that will provide research support (mentorship), and the support that will be provided (including the reason for the choice).
Collaborations
Please use this section to detail the establishment of new collaborations, or the development of existing collaborations that will result from time with the NCI Host research groups in the United States.
- Applicants should detail the collaborations they intend to establish or develop over the course of the award.
- Applicants should detail what the collaborations will involve and the added value (e.g. access to facilities and expertise).
- Applicants should detail why these collaborations will be beneficial for their career and how the award will enable these collaborations to be developed.
A statement of support from the NCI Host should also be added separately as a collaborator letter in the 'Uploads' section.
Host Organisation Support Statement
The statement is completed by the Head of Department and should detail how the Host Organisation is going to support the applicant to successfully complete their training and development programme as detailed in this application. This statement should be tailored specifically to the applicant, their training needs, and include how the organisation intends to support the applicant to develop their research career in the long-term.
NIHR sees the responsibility for training the next generation of research leaders as a joint enterprise with Host Organisations. Therefore information should also be provided on the organisation’s track record of supporting early career researchers, such as evidence of bridging or other support provided to fellows upon completion of an award.
In addition, the statement should also describe the Host Organisation’s approach to creating and maintaining an inclusive and supportive research culture for all. The statement should provide evidence of how the organisation values and supports equality, diversity and inclusion as well as acknowledging the organisation’s responsibilities with respect to research integrity. Statements may wish to refer to the principles and best practice outlined within relevant Charters and Concordats in these areas, such as the Researcher Development Concordat and Advance HE’s Equality Charters. It should be noted that being a signatory to Concordats or holding bronze/silver status from the Equality Charters isn’t a requirement of funding and evidence can be provided through other means.
Host Organisations are expected to comply with the relevant Principles and Obligations for clinical academic training and it is recommended Host Organisations read these documents, where relevant, before completing the statement of support.
NIHR expects that all commitments made to the applicant within this statement will be honoured for the lifetime of the award.
5. Detailed Budget
Justification of costs
This section should be completed in conjunction with your Host Organisation’s finance office. You should only include the funding requested for this award.
Please provide a breakdown of costs associated with undertaking the award and provide justification for the resources requested.
This award provides funding for:
- The salary of the award holder for 6-12 months
- Travel, accommodation* and subsistence costs associated with visits tothePartnerNCI in the United States
- Includes both personal and family relocation costs
- Includes visa costs and health insurance
- Training and development costs up to £5,000
- Conference costs up to £2,000
- Research costs and consumables
- NCI partner research groups will support standard consumables, but where substantial or exceptional additional research costs are required for the proposed research and skills development, costs may be requested where justified.
- Funding for clinical trials can not be requested, however, costs for collecting pilot data and conducting preliminary work to support future research funding applications can be included.
*Please note - For stays exceeding 2 months, applicants are encouraged to explore cost-effective accommodation options.
All costs will be paid at 100%. Full Economic Costings (FEC) are not covered by this award.
This award is not a project or programme grant; therefore, extensions to the duration of awards to allow for completion of training and development are not permitted. This doesn't affect suspensions of awards to allow for periods of maternity, paternity, adoption or sickness leave.
Detailed Budget Breakdown
The finance section should provide a breakdown of costs associated with undertaking the research as described in the proposal.
General Information
The information entered in this section should provide an analysis of the total funds requested to undertake the activity proposed and should be based on current prices. These costs will be used to assess value for money.
It is in the best interest to undertake a thorough, realistic and accurate costing. You must provide a clear and full justification for all costs.
Costs must be provided at current prices. An adjustment for inflation will be made annually thereafter at rates set by DHSC. Whilst allowances for incremental increases should be included on the form, nationally or locally agreed pay increases should be excluded.
Further itemisation of costs and methods of calculation may be requested to support the application at a later date.
Payments will be made to the contracted organisation only and the contracted organisation will be responsible for passing on any money due to their partner organisation(s).
Appropriate sub-contracts must be put in place for any element of the research which is to be paid to another organisation.
All applications are expected to have appropriate input from the Host Organisation into the finance section of the application form.
Information on Different Types of Organisations
This award does not cover Full Economics Costs therefore all prices should be entered at, and will be paid at, 100% regardless of the type of organisation (NHS, HEI or Other).
If the Employing host organisation is a Higher Education Institution, please select the “other” option when entering the “Type of Cost” to prevent costs being submitted at 80%.
Direct Costs
These are costs that are specific to the award, which will be charged as the amount actually spent and can be supported by an audit record. They should comprise:
Details of posts and salaries
This section presents an overview of salary costs for the applicant, including normal salary current increments broken down individually.
Applicant
Please state the proposed salary point and scale at the start of the award. Please note immediate promotion to a higher grade as a result of securing a fellowship will not be funded.
The Apprenticeship Levy can be included in the salary costs from 1st April 2017 where relevant.
1) Salary costs
This section specifies the annual costs of the applicant. You should now allocate the individual staff member costs to each year of the award, allowing for increments. Use current rates of pay, and build in any known annual increments (again at current rates). You will not be able to claim for pay awards retrospectively, once your fellowship is underway.
Please note the salary figures need to be calculated using the current annual costs, %WTE and number of months.
It is important to double check that the %WTE, total months and yearly costs information are consistent with the information presented in ‘Details of Posts and Salaries’ (‘Details of Posts and Salaries’ should show the full current staff costs independent of % WTE etc., whereas the yearly costs in ‘Salary Costs’ depend on % WTE etc.).
2) Travel, Subsistence and Conference Fees
When detailing your budget for travel, accommodation, and relocation costs related to your visit(s) to the NCI Host, please ensure that you include all relevant expenses in this section. We recognise that, for various reasons, the inclusion of family members can be crucial and is therefore an allowable cost.
When justifying a travel budget, we expect applicants to prioritise cost effective options to maximise best value for the resources available.
3) Training and Development
Please itemise and describe fully the costs associated with training and development. Please provide estimates if exact costs are not available at the time of application. Any travel and subsistence associated with training and development should not be included here and should be included in the travel section of the form.
4) Other Costs
Please use this section to justify any other costs that are required as part of your training and development programme.
NCI Hosts will support standard consumables.
Please note: Although CRT-DSE award holders may undertake some research during their placement, this award does not provide funding to undertake a research project. Exceptional research costs may be requested if applicants can demonstrate that these will contribute to their development.
6. Uploads
To support your proposal you are required to upload the following documents in the ‘uploads’ section of the form:
- Collaborators letter of support (required): Please include a letter(s) from the NCI Host
- Training & Development Gantt Chart (Required): Please include a Gantt chart which details the activities that will be undertaken as part of this award to aid the selection committee members in understanding the timeline.
- Letter of support where research involves human participation / human tissue / animals. Please complete the ‘MRC use of animals overseas’ form, if applicable.
- If you have applied unsuccessfully for a CRT-DSE previously, please upload a cover letter which details how this application differs and how any feedback received has been used to inform this application.
7. Participants and Signatories
A number of participants and a signatory are required to be added to your application and, where applicable, to complete sections of it. Details of the required individuals are provided on the online application form along with details of how they should be added.
8. Acknowledge, review and submit
Conflict checks
Please declare any conflicts or potential conflicts of interest that you may have in undertaking this research, including any relevant, non-personal & commercial interest that could be perceived as a conflict of interest. Please check with your Host Organisation if unsure.
Agreement to terms and conditions
Please click the check box to confirm you agree to the Terms and Conditions of submission as detailed on the application form.
Completing and submitting the form
Please see Annex B for flow diagrams of the application submission process.
Applicant:
You will need to complete all of the mandatory sections of the form and enter under the ‘Participants and Signatories’ section the names and contact details of participants and a signatory (see below). Once all other parties have made their contribution, you will be required to ‘Submit’ the application to the signatory for final sign off before the closing date. Please note that you will need to read and be aware of the roles of participants and signatory as described in these guidance notes.
You will only be able to press the ‘Submit’ button, which will send the application for final sign off by the signatory when:
- all mandatory sections of the application form are complete;
- all participants have agreed to be part of your application;
- the signatory has agreed to their role;
- The Head of Department has completed the ‘Host Organisation Support Statement’.
Please note; when completing the application form, you are advised to validate your application as you go. You will find a Validation Summary button in the left hand menu. This section will detail any points within your application that are either incomplete or incorrect. Failing to validate your answers may result in you being unable to submit your application by the required deadline.
Participants:
You are required to supply the names and email addresses (if not already registered on the ARAMIS application system) of the individuals who will be undertaking ‘participant’ roles as part of your application. Everyone named in this section will be acting as a ‘participant’ to your application and will need to agree to be part of this application. Participants are required to review the declaration for the role before confirming participation as part of the one-click ‘confirm’ process.
By confirming participation, participants are acknowledging their involvement and input into this application and agree to be involved in it before it is submitted. You must ensure all participants are happy for your application to be submitted before submitting it on the online system.
Required Participants (if applicable)
- Research Support: The individual(s) providing uk-based Research Support (mentorship) must confirm that they have read the application and the guidance notes and are willing to act as your mentor for career development and agree to abide by the conditions under which an award may be granted. If you have included external collaboration in your application for example with a CTU, Industry or Health data science they must confirm their participation in this section of the application.
- PhD Primary Supervisor: If you have submitted your thesis but have not yet been awarded the degree, your primary supervisor needs to be included in the application to confirm this. (Signatory on ARAMIS is Post-Doctoral Primary Supervisor)
- Host Organisation Administrative Authority or Finance Officer: The Administrative Authority or Finance Officer for the Host Organisation must confirm that they will ensure the accuracy of the financial details of the application and that the Host Organisation is prepared to host and administer the award, at the stated costs, if made.
Participants must confirm their participation on your application before you will be able to press the submit button. They will have no further action to take in the submission process once you have submitted. It is recommended that you contact your participants as early as possible to ensure they understand any action they must take before you can submit the application.
Signatory
You are required to supply the names and email address (if not already registered on the ARAMIS application system) of the individual who will be ‘signing off’ your application. Once their contact details have been entered, the signatory will be invited to log into the system and confirm their participation. Details of what is required and expected of the role can be found below.
The signatory will be required to agree to the role being asked of them in the application before the application is submitted by the applicant, and then approve the final version of the application after it has been submitted via the online system, i.e. they must have agreed to participate and complete their section before the applicant is able to press the SUBMIT button and send the application for signatory approval.
The Signatory must approve the application after the applicant has selected the SUBMIT option but BEFORE the application submission deadline.
Please see the 'Application Submission Process Flow Diagram’ (Annex B) for further information. The final signatory approval will result in the application being fully submitted to the NIHR. All parties (applicant, participants and signatory) will be notified of this via an automated system generated email.
NIHR will not accept any applications unless fully approved by your signatory prior to the 1pm deadline, no exceptions will be made.
Required Signatory:
- Head of Department or Senior Manager: You will be required to include the Head of Department from your Host Organisation. The Head of Department from the Host Organisation (in which this award will be based) must confirm that they support this application and that, if funded, the research and training will be supported and administered in the named organisation and that the applicant for whom they are responsible will undertake this work. As such, the Head of Department will be required to complete questions in the ‘Training & Development and Research Support’ section of the Application Form.
Once the application is ready (see list of required steps under the ‘applicant’ heading above), you will be able to ‘Submit’ the application for final sign off by the signatory. At this point, the signatory will be prompted to log back into the system and approve the finalised application. The application will not be submitted to the NIHR for consideration until the required signatory has approved the final version. When the Head of Department or Senior Manager presses the approve button, the application will be submitted to NIHR.
Please note that all the steps described here need to take place before the deadline of 1:00 pm on the final day of the submission window, no exceptions will be made.
Should you require assistance in completing the online form, please contact the NIHR Academy at 0113 532 8410 or by email.
Additional Supporting Information
Plagiarism in NIHR funding applications
NIHR and UKRI expects all content within applications for funding to be original material of the applicant's own work, with the exception of sections that other participants are required to complete. Whilst we anticipate and expect that applicants will get help and advice from various sources when putting together an application, including on occasion input from those previously awarded funding, care must be taken to ensure this does not lead to plagiarism of either published work or other previous applications. If an allegation of plagiarism is raised against an application this will be investigated in accordance with the NIHR Academy’s policy on plagiarism, a copy of which is available on request from academy-awards@nihr.ac.uk.
NIHR Privacy Policy
Our purpose for collecting information is to communicate with you about your application and have the necessary information to evaluate you for a fellowship. The data we collect here is collected in the public interest. Information provided here may be subject to Freedom of Information requests.
The NIHR Academy is part of the Department for Health and Social Care (DHSC), National Institute for Health Research (NIHR). The contracting agent for the NIHR Academy is the Leeds Teaching Hospital Trust (LTHT). The DHSC is the Data Controller and LTHT is the Data Processor under the General Data Protection Regulation (GDPR) EC 2016/679. DHSC NIHR respects the privacy of individuals who share their data and processes it in a manner that meets the requirements of GDPR. The DHSC Data Protection Officer can be contacted by email at data_protection@dhsc.gov.uk.
The NIHR privacy policy includes further information including ways we may use your data, our contact details and details on your individual rights regarding how your data is used. Your data may be shared across the NIHR, MRC – UKRI, NIH and with the Department of Health and Social Care, including with other coordinating centres, to allow the application to be managed and for statistical analysis, and with external grant reviewers as part of the process for managing the allocation of a grant. Information collected from you will not be shared outside the EEA without your consent.
This notice is under constant review and will be updated and / or revised based on that review as appropriate.
Equality and Diversity Monitoring Information
NIHR is committed to promoting equality, diversity and inclusion in research and asks applicants to provide Equality and Diversity Monitoring Information (age, sex, ethnicity and race, and disability). By answering these Equality and Diversity Monitoring Information questions, you will help us to better understand the different groups of people that we fund and their experiences of being funded – particularly the groups protected by UK equality legislation. Although it is mandatory to answer these questions, it is possible to select “prefer not to say” as a response. However, the more information you provide, the more effective our monitoring will be. This information will not be used to make decisions about funding.
NIHR Carbon Reduction Guidelines
Researchers applying for NIHR funding are asked to consider the carbon footprint of their research and take steps to reduce carbon emissions where appropriate. Advice on how to do this can be obtained from the NIHR Carbon Reduction Guidelines.
Transparency Agenda
In line with the government’s transparency agenda, any contract resulting from this tender may be published in its entirety to the general public. Visit the GOV.uk website for further information on the transparency agenda.
Contractual Arrangements
Financial support under an NIHR Fellowship is subject to a contract between the Department of Health and Social Care (DHSC) and the host organisation.
Once funding for this award has been discussed and agreed, NIHR will confirm the financial arrangements with the host organisation. NIHR will provide the host organisation with a contract setting out the details of these arrangements.
The host organisation will be expected to issue the individual with an employment contract commensurate with their experience and seniority.
Government procurement transparency regulations require publication of details of all contracts made with the DHSC on their Website. Confidential information including research proposals (Plain English Summaries will be published), detailed finance information, bank details, and departmental staff names (other than the award holder’s name) will be removed from the published versions.
Freedom of Information Act
The NIHR Academy manages the NIHR Fellowship Programme on behalf of the DHSC. As such the findings of researchers funded by the programme are incorporated in to the Department of Health and Social Care Freedom of Information Publication Scheme.
Equal Opportunities and Diversity
NIHR and UKRI are committed to promoting equality, diversity and inclusion in research. NIHR asks applicants to provide Equality and Diversity Monitoring Information (age, sex, ethnicity and race, and disability). By answering these Equality and Diversity Monitoring Information questions, you will help us to better understand the different groups of people that apply to us for funding and their experiences of the funding process – particularly the groups protected by UK equality legislation. Although it is mandatory to answer these questions, it is possible to select “prefer not to say” as a response. However, the more information you provide, the more effective our monitoring will be. This information will not be used to make decisions about funding.
Guidance and Advice
Please read these Guidance Notes carefully. If you require any further information, advice or guidance please contact the Personal Awards team by calling 0113 532 8410 or email academy-awards@nihr.ac.uk.
Ethics / Regulatory Approvals
Guidance on the application process for ethical and other approvals can be found on the HRA website. Please note that if your study is led from England and involves the NHS in England you should apply for HRA approval.
If you are using patient information from an existing database, you should check whether the patients have given their consent for their data to be included in that database for research purposes, or if not whether the database is exempt under Section 251 of the NHS Act 2006. Where exemptions are not already in place, approval to use confidential patient information without consent must be requested from the HRA who make decisions with advice from the Confidentiality Advisory Group (CAG).
Note: NIHR is interested in taking advantage of the growing utility of routine data (such as HES, GP records etc.), and would like investigators, where appropriate, to ask study participants to consent to long term follow up (e.g. beyond the outcomes to be collected in the funded trial) using routinely collected data, and appropriate linkage to allow this data to be best used.
Annex A: Additional UKRI Requirements
Human participation / use of human tissue
A signed and dated letter of support must be attached when human participation / human tissue research is proposed. The letter must be signed by both the applicant and a representative from NCI host.
The letter should state that applicants will comply with the relevant MRC – UKRI policies and guidance in the standard MRC – UKRI Guidance for Applicants and should also acknowledge that the applicant and NCI host understand that MRC – UKRI’s current policy for research involving humans to take place overseas, is that for research to be undertaken internationally, both local and UK ethical approval is required. The letter should also state that the applicant and NCI host understand that for clinical studies involving human participants and/or patients in the UK or overseas, appropriate consent must be obtained.
In addition, where the NCI partner or another third party (any organisation other than the applicant’s UK host) is responsible for recruitment of people as research participants and/or providing human tissue, details should be included in the application and the letter of support must include confirmation of the following:
- which international partner is involved and that the partner has agreed to recruit the participants/provide tissue
- that what is being supplied is suitable for the research being undertaken
- that the quantity of tissue (where relevant) being supplied is suitable, but not excessive for achieving meaningful results.
Use of stem cells
Please see section 5 of the standard MRC Guidance for Applicants for further information.
Use of animals
All applicants are required to comply with Section 4: ‘Proposals involving animal use’ of the standard MRC Guidance for Applicants and applicants must ensure that all of the proposed research, both that in the UK and in the US will comply with the principles of the MRC common guidance on responsibility in the use of animals in bioscience research. Applicants should attach the MRC ‘Use of Animals Overseas’ form(s) as an additional document - please see the MRC Guidance for Applicants and the use of animals overseas section of the National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs) website.
Investigators proposing the use of animals (in either country) should read the guidance, which includes information on research or collaborations outside the UK and:
- provide a dated letter which must be signed by both the applicant and a representativefromtheNCI host stating that:
- research will adhere to all relevant national and local regulatory systems in the UK and the US
- they will follow the guidelines laid out in the responsibility in the use of animals in bioscience research. document and ensure that work is carried out to UK and US standards. If primates are used they should also confirm that they will follow the NC3Rs Guidelines: Primate Accommodation, Care and Use
- before initiation of the proposed research work, appropriate approvals from institutional and/or central animal ethics committees will be obtained for experimental protocols to be adopted in their projects. Successful proposals may be expected to provide copies of these permissions before funding is released.
Trusted Research and Innovation
UKRI is committed to ensuring that effective international collaboration in research and innovation takes place with integrity and within strong ethical frameworks. Trusted Research and Innovation (TR&I) is a UKRI work programme designed to help protect all those working in our thriving and collaborative international sector by enabling partnerships to be as open as possible, and as secure as necessary. Our TR&I Principles set out UKRI’s expectations of organisations funded by UKRI in relation to due diligence for international collaboration. As such, applicants for UKRI funding may be asked to demonstrate how their proposed projects will comply with our approach and expectation towards TR&I, identifying potential risks and the relevant controls you will put in place to help proportionately reduce these risks.
Further guidance and information about TR&I – including additional where applicants can find additional support – can be found on UKRI’s website.
Annex B: Application Submission Process Flow Diagram
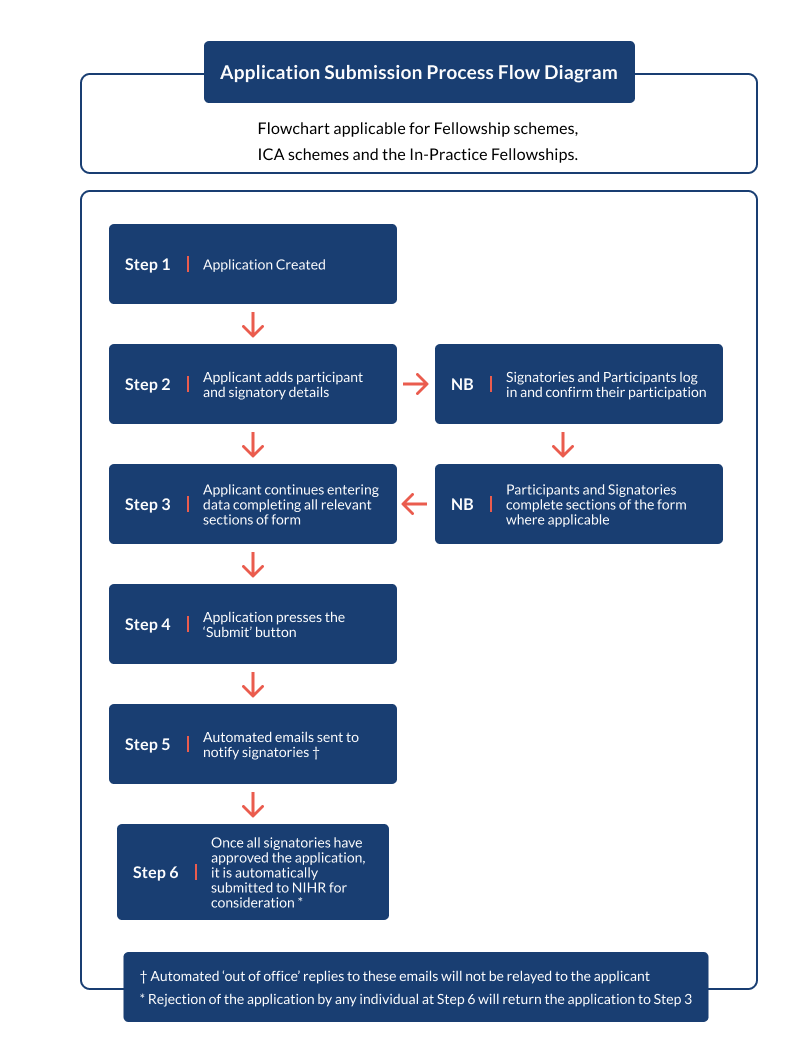
Outlined above are the steps for submitting an application. The applicant starts the application and adds participant and signatory details. The participants and signatories can then log in and confirm their participation and signatories can complete the sections of the form as directed. The applicant can continue entering data and completes all relevant sections of the form (step 3).
The applicant then presses the ‘Submit’ button. Once the applicant submits, signatories will receive automated emails to approve the application. However, automated ‘out of office’ replies to these emails will not be relayed to the applicant.
Once all signatories have approved the application, it is automatically submitted to NIHR for consideration. Rejection of the application by any individual at this stage will return the application to step 3.

