On this page, you will find information about our HealthTech Research Centres (HRCs), which work with medical device, diagnostic and digital health technology companies to develop, evaluate and validate new medical technology and diagnostics.
Help to develop your medical device or technology for the NHS
HRCs are centres of excellence located in leading NHS organisations across England that accelerate the development of healthcare technologies to improve the effectiveness and quality of health and care services.
The HRCs support the development of innovations that address the most pressing healthcare challenges, including cancer, mental health, neurodegeneration and dementia, ageing, respiratory disease, and cardiovascular conditions.
Our HRCs can help medical device, digital technology and diagnostic companies (collectively known as HealthTech) to develop new innovative medical technologies. This includes help to generate the evidence to demonstrate financial value (health economics) or improve operational efficiency in the NHS (real-world evidence generation).
We also help non-life science companies, such as software developers and digital companies, that are working on healthcare solutions
We have established 14 HRCs, each with a distinct therapeutic focus, to bring together the life sciences industry, patients, carers, the NHS, researchers, commissioners and investors. Through our HRCs, we can provide:
-
Expertise
Unrivalled access to world-leading medical diagnostics expertise and extensive clinical networks.
-
State-of-the-art facilities
Access to specialist equipment relevant to distinct specialisms such as a sleeping unit, a surgical theatre simulation suite, a gait analysis lab and a neuroimaging suite.
-
Advice
Advice on the suitability of new technologies and the evidence required to support their commercial uptake. This includes advice on device design, patient care pathway analysis, clinical evaluation, health economics, site identification, patient recruitment and regulatory advice.
-
Collaboration
Help to broker collaborations between industry and clinical practice to test new technologies in community settings. We can help you to build a multidisciplinary team to work collaboratively with you to ensure you generate the evidence you need to support the adoption of your medical technology into the NHS.
-
Patient and public involvement
Public contributors are at the centre of our HRCs, helping to steer and govern our work to ensure it is relevant to patients and carers. Each HRC has a PPIE lead to support companies in their product design, development and to support patient engagement and recruitment.
-
Study delivery
In-house delivery of clinical trials, health economics, health informatics, multidisciplinary pathology capabilities, and generation of high quality evidence to demonstrate the benefits of your product.
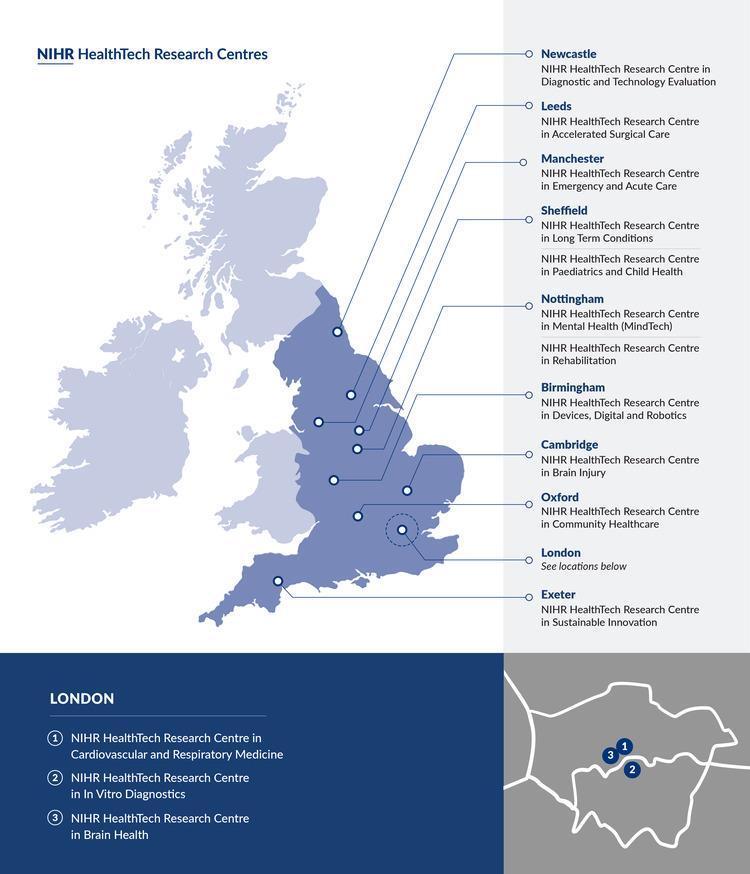
The NIHR HRCs launched on 1 April 2024, with more than £41 million awarded across 14 NIHR HRCs over 5 years. The HRCs are shown in the map on this page and are listed here:
- NIHR HealthTech Research Centre in Accelerated Surgical Care
- NIHR HealthTech Research Centre in Brain Health
- NIHR HealthTech Research Centre in Brain Injury
- NIHR HealthTech Research Centre in Cardiovascular and Respiratory Medicine
- NIHR HealthTech Research Centre in Community Healthcare
- NIHR HealthTech Research Centre in Devices, Digital and Robotics
- NIHR Healthtech Research Centre in Diagnostic and Technology Evaluation
- NIHR HealthTech Research Centre in Emergency and Acute Care
- NIHR HealthTech Research Centre in In Vitro Diagnostics
- NIHR HealthTech Research Centre in Long Term Conditions
- NIHR HealthTech Research Centre in Mental Health (MindTech)
- NIHR HealthTech Research Centre in Paediatrics and Child Health
- NIHR HealthTech Research Centre in Rehabilitation
- NIHR HealthTech Research Centre in Sustainable Innovation
The HRC scheme replaces the successful NIHR Medtech and In Vitro Diagnostic Co-operative (MIC) scheme, which came to an end in March 2024.
MIC impact
The NIHR MIC scheme, which ran from 1 January 2018 to 31 March 2024, included th e development of:
- Virtual reality rehabilitation physiotherapy for children. This enabled them to undergo treatment from the comfort of their own home, reducing pressures on hospital services;
- The “HeadUp Collar”. This drastically improves the quality of life for people with motor neurone disease. It addresses problems with communication, swallowing, breathing, mobility and pain;
- A breath test for multiple gastrointestinal cancers. This enables detection at an earlier stage, when treatments are more effective;
- QbTest, a computerised assessment of attention deficit hyperactivity disorder (ADHD). This is now used in NHS ADHD Clinics in England to support clinical decision making and more efficiently diagnose ADHD. QbTest enables people to have fewer consultations and receive support much earlier.
If you are interested in working with the NIHR HRCs or would like more information, please get in touch with us.


