Eligibility
Is my organisation eligible to apply?
We are keen to hear from all organisations that have an innovation in opioid and cocaine addiction and recovery healthcare which is beyond concept stage.
Are only health organisations able to apply as it is being funded through NIHR?
No - organisations from any sector can apply to the fund, as long as they are a UK legal entity. We are working with NIHR and the Office for Life Sciences to identify a broad range of independent experts from across different sectors to assess applications at each phase.
What if my organisation is new to research?
We recommend that organisations which do not have an established R&D function apply in partnership with a researcher or research organisation.
Organisations and individuals looking for potential collaborators can receive support from the NIHR Business Development Team. Email them at Industry@NIHR.ac.uk, including a short description of the proposed intervention to be developed, the type of collaborators sought (such as academics, practitioners or community-based organisations) and the kind of contribution needed from those collaborators. The Business Team then looks to match up appropriate organisations to discuss ideas.
If you need any help with defining your ideas and preparing your research plan and application, please contact the NIHR Research Design Service for free help. Please note that the RDS service is only available to applicants based in England.
I need help with research design, what if my organisation is based in Scotland, Wales or Northern Ireland?
Please note that the RDS service is only available to applicants based in England. Applicants with a project partner based in England are able to access the service, but if you are based in the UK but outside of England, and are not partnered with an organisation based in England you may wish to explore the below services. The NIHR Business Development Team is available to all UK based applicants and you may wish to contact them in seeking a partner based in England.
- Applicants from Scotland may access The Drugs Research Network Scotland
- Applicants form Northern Ireland may access The Northern Ireland Clinical Research Network
- Applicants from Wales may access Health and Care Research Wales
What if my organisation is based outside of the UK?
The lead applicant must be based in the UK and, if found to be successful, the proposed research must take place within the UK and the intervention must be deliverable in UK nations. It is fine to have international partners and indeed outcomes for international patients, but the focus of the work and its outcomes must be for UK patients.
Are government departments and devolved administrations eligible to apply?
Yes. The fund is open to applications from any UK organisation that is a legal entity and has a credible proposal for reducing drug demand. However, it cannot be used to provide additional funding for activities that are already being financed through other existing local or national budgets.
Can international co-applicant/collaborators be included as part of a project team?
Yes, involvement of international companies as subcontractor and/or collaborator are welcomed although it must be justified. However, the lead applicant must be based in the UK and, if found to be successful, the proposed research must take place within the UK and the intervention must be deliverable in UK nations.
My organisation already holds funding from the Department of Health and Social Care (DHSC), or is a strategic partner of the Department of Health and associated departments, are we eligible?
If you already hold funding from the DHSC, you may still be eligible as long as your proposal to the AMI Awards does not constitute any doubling up of efforts of funding. If your proposal to the AMI is entirely separate from your current funding, you may still be eligible. Please contact us to discuss this further.
If your organisation is a strategic partner of the Department of Health, or associated departments you are unlikely to be eligible. Please contact us to discuss this further.
My organisation has its own research contracting process, are the terms of the NIHR Research Contract negotiable?
No. Applicants must review the NIHR standard research contract before application submission, and agree in principle with its core terms and conditions as they are non-negotiable.
Am I eligible to apply?
If you would like to act as lead applicant, you must apply through the affiliated organisation you belong to. The call is open to any organisation that is a UK Legal Entity. Proposals led by individuals who are not affiliated to a legal entity are not eligible.
What if I am new to research or early on in my career? Can I apply?
To support the growth of innovators in this field, applications from early career researchers and innovators are particularly welcomed. You can apply as either the lead or joint-lead applicant together with a senior colleague fulfilling the other role. We are keen to encourage fresh ideas from new innovators and appropriate applications are welcomed from those with limited research experience when supported by an experienced, strong and multi-disciplinary team.
Is there a limit to the number of applications any one person or organisation can submit or be part of the project team for?
NIHR will not accept the same or substantially similar applications to more than one NIHR programme. If two similar applications are submitted, once the overlap is identified, the application that is most advanced through the funding process will continue and the second will not be taken further.
Similar applications will only be considered by two programmes concurrently if:
- The aims and research proposals are substantially different
- If successful, NIHR would be prepared to fund both proposals
- The successful delivery of one project is not dependent on the other.
Is my proposal eligible for funding?
Proposals are welcome for innovations at the majority of development stages. Please see the schematic below which illustrates the eligible entry points for this call.
Although, strictly speaking, a demonstrated proof of concept is not required, the most competitive proposals are expected to show data and evidence to support the case for further development.
Basic research projects are out of scope.
Digital and MedTech/Non-pharmaceutical innovations must be beyond concept stage, and ideally have a prototype ready to test outside the laboratory. This is outlined in the schematic as TRL-4 and beyond. Prototype development, however, is acceptable as part of the initial stages of the proposal but this must be beyond the conceptual stages. Eligibility is outlined as TRL-3 and above. If you are yet to develop your prototype and are unsure if you meet TRL-3may warrant discussion with please contact the AMI Support Team for advice– see TRL-3.
Pharmaceutical innovations evaluated by clinical trials are eligible from Phase One (first-in-human), with the exception of Phase Three trials. Phase Three trials are not eligible due to their size and expense, however eligible adjuncts to existing Phase Three trials are welcomed. Please contact the AMI Support Team to discuss whether your Phase Three adjunct project is eligible.
Entry Point Eligibility Schematic
Entry points in red are out of scope, those in blue are within scope. If your project falls within an orange entry point, please do contact the AMI support team to discuss eligibility.
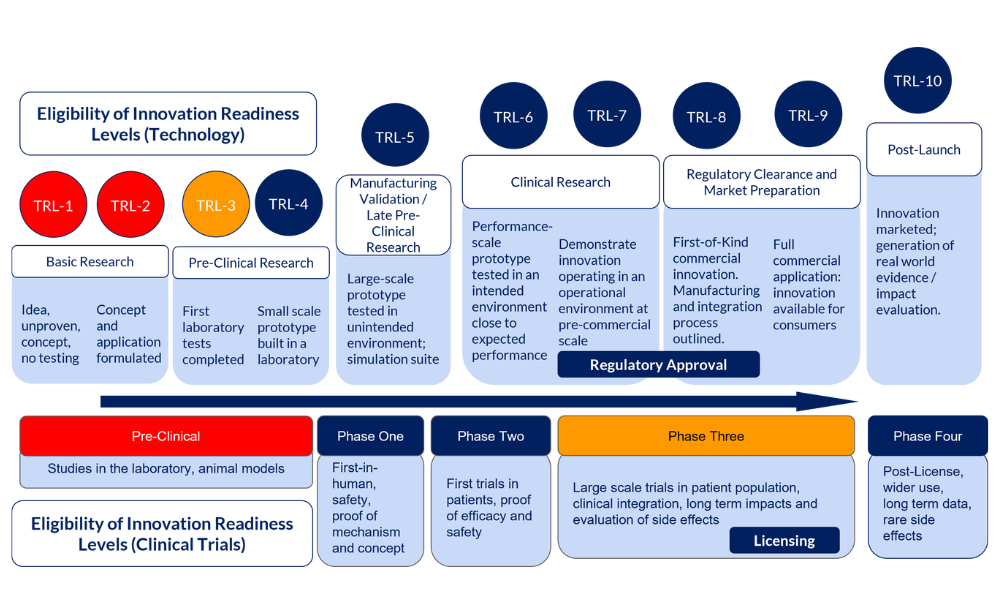
Entry Point Eligibility Schematic Description:
If your innovation is technology: Technology Readiness Levels (TRL)
- Not eligible (red)
- Basic Research
- TRL-1: Idea, unproven, concept, no testing
- TRL-2: Concept and application formulated
- Eligible (blue)
- Available data/evidence to support the case for further development
- Pre-Clinical Research
- TRL-3: First laboratory tests completed
- TRL-4: Small scale prototype built in a laboratory
- Manufacturing Validation / Late Pre-Clinical Research
- TRL-5: Large-scale prototype tested in unintended environment; simulation suite
- Clinical Research
- TRL-6: Performance-scale prototype tested in an intended environment close to expected performance
- TRL-7: Demonstrate innovation operating in an operational environment at pre-commercial scale, gaining regulatory approval
- Regulatory Clearance and Market Preparation
- TRL-8: First-of-Kind commercial innovation. Manufacturing and integration process outlined, gaining regulatory approval
- TRL-9 :Full commercial application: innovation available for consumers
- Post-Launch
- TRL-10: Innovation marketed; generation of real world evidence / impact evaluation.
If your innovation requires a Clinical Trial: Clinical Trials
- Not eligible (red)
- Pre-Clinical: Studies in the laboratory, animal models
- Phase Three: Large scale trials in patient population, clinical integration, long term impacts and evaluation of side effects, licensing
- Eligible (blue)
- Phase One: First-in-human, safety, proof of mechanism and concept
- Phase Two: First trials in patients, proof of efficacy and safety
- Phase Three Adjuncts: Eligible adjuncts to an existing, current Phase Three Clinical Trial
- Phase Four: Post-License, wider use, long term data, rare side effects
What do you mean by no upper funding limit?
There is no upper funding limit for proposals. We would expect the majority of applications to request between £500,000 and £1.5m funding, however applicants are discouraged from compromising their ideas to meet this guideline, and applications will be considered on the overall merit and value for money they provide.
I have an addiction/recovery psychological intervention, am I eligible to apply?
Projects which propose a novel way to deliver existing psychological interventions through digital technology are within scope. Novel psychological interventions themselves are not and the NIHR have a number of other funding streams that may fit your project better.
I have an addiction/recovery education intervention, am I eligible to apply?
The focus of the AMI awards is for innovators to secure support for research and development of innovative digital, MedTech, and pharmaceutical interventions that improve treatment and recovery for substance misuse patients. Your proposal may have an element of education, but innovations focussed solely around education are not within scope.
I have an innovation in detecting/treating/preventing overdose events, am I eligible to apply?
No. The OLS Addiction Mission in partnership with the Scottish Health Industry Partnership (SHIP), launched a separate £5m Reducing Drug Deaths Innovation Challenge which aims to develop innovative MedTech and Digital Health solutions that focus on detection of, response to, and intervention in, potentially fatal drug overdose episodes.
I have an innovation in preventing addiction, am I eligible to apply?
No. The AMI Awards are solely focused on innovation in addiction and recovery treatment - pre-addiction initiatives are out of scope.
The Innovation Fund to Reduce Demand for Illicit Substances (RDIS) Phase Two call is currently welcoming applications to evaluate novel methods of reducing so-called recreational use of illicit substances.
Would my proposed intervention have to be piloted in England?
No. Interventions can be piloted anywhere in the UK. Proposals are encouraged to plan towards their innovation being feasible for uptake in as many UK Nations as possible.
My proposal contrasts current government proposals and policy, am I eligible for this programme?
Any projects that are implemented at the end of the programme would need to align with government policy and approach regarding illicit substances. If you have a proposal that contrasts the government’s current policies and approaches, an alternative funding programme within NIHR may be more appropriate. You can find information about other funding opportunities here.
Why must I have a PPI Lead costed into my project? Are they full time?
It is to be expected that the Patient and Public Involvement (PPI) members working with your project, as either participants or as members of the research team, may be vulnerable adults, with current or lived experience of substance abuse and addiction. To ensure the safety and support of participants, or members of the research team, who have current or lived experience of drug use, addiction and/or recovery, it is expected that proposals include the time of a PPI Lead to support potentially vulnerable people involved in this work. This person need not be full time - just available to consult and provide support as necessary.
The PPI Lead should be a named person with appropriate skills and experience who is responsible for leading the PPI element within the project. The PPI Lead should be an adequately costed and resourced research team member who is able to manage the PPI plans and related activities. More information and examples of the activities a PPI lead might undertake can be found in our guidance on the NIHR website.
Safeguarding is everyone's responsibility, it is expected that this will be considered in your application.
The Application Process
How do I apply?
You must apply using the online form in the Research Management System (RMS), which is available from 31 May 2023.
If your organisation has not applied for NIHR funding before, you will need to register your organisation on the Research Management System, and have received validation notification, before you will be able to access the application form.
Please ensure that you have registered your organisation, and your co-applicants organisations, well before you attempt to submit your application.
Why are there two stages to the application process?
A two stage application process enables the independent committee to offer advice on the stage 1 application and idea before it goes on to a full stage 2 application. This ensures that all applications invited to stage 2 are well developed ahead of consideration for funding. This also means that the final projects selected for funding to be in the best possible place to get the most out of the evaluation. This approach also minimises the time investment of teams whose proposals are least likely to be successful or who are not eligible.
The application window for Stage 1 feels short, is this definite?
Stage 1 is a very short application process, and the time allowed reflects that. There are six weeks to apply at Stage 1, the deadline of 13:00 on 12 July 2023 will not change. This timeline is set in order to take advantage of the funds available.
Stage 2 is open from 23 August 2023 to 11 October and this gives good time to outline the full details of your research plan, team and financial request.
Do service providers need to partner up with a research lead before submitting an expression of interest, or could we use that process to find one? How can we link up with researchers for our applications and proposed projects?
The NIHR has a system that enables organisations to be put in touch with researchers, and vice versa, to discuss ideas. There is no guarantee that a match will be successful, however, organisations and individuals looking for potential collaborators can email the NIHR Business Development Team at industry@nihr.ac.uk, including a short description of the proposed intervention to be developed, the type of collaborators sought (such as academics, practitioners or community-based organisations) and the kind of contribution needed from those collaborators.
It is advised that you do this at the earliest opportunity.
When will I hear whether my application has been successful?
The call for Stage 1 applications will close on 12 July 2023. The proposals will then be assessed by an independent committee made up of experts. We are aiming to inform applicants of the outcome of their Stage 1 applications early in August 2023. Stage 2 will open to those successful at Stage 1 on 23 August 2023 and will close on 11 October 2023. Projects must start between 26 February 2024 and 11 March 2024, following successful due diligence and contracting processes.
If my project is unsuccessful, are there any other funding pots I can apply to?
You may wish to consider applying to other funding opportunities run by NIHR.
Background and Scope of the Fund
Are any other drugs in scope? Could bids focus on other drugs that are controlled under the Misuse of Drugs Act? What about drugs that are not controlled, such as weight loss drugs? What about alcohol and tobacco?
Innovations must focus on opioid or cocaine addiction. Innovations that are also applicable to other substances would be in scope only if they:
- primarily target opioid or cocaine addiction, and the focus of the proposed research project is to help treat people with opioid or cocaine addictions and aid in their recovery.
- support treatment or recovery for people with a pattern of polydrug use centred on opioid or cocaine addiction.
Why are you looking at only addiction recovery? What about recreational use?
This call aims to encourage innovation in the treatment of, and recovery from, opioid and cocaine addictions.
There is other funding available for recreational use research, such as the NIHR Innovation Fund to Reduce Demand for Illicit Substances (RDIS) which is running alongside this call. RDIS however is not focused on the recreational use of opioids or crack cocaine as there is already wider available funding and work focusing on the pre-addiction stages of opioid and crack cocaine use.
Would the funding call also focus on building up sector capacity and skills (including skills for commissioning of prevention), or is the focus primarily on 'interventions'?
The main aim of the call is to encourage innovation and to enhance the UK-wide research environment and incentivise the development of innovative and effective treatments, technologies, and approaches to support recovery, and reduce the harm and deaths which substance addictions can cause.
We would therefore consider bids containing innovation to develop and mobilise the workforce, and where it is clear that evidence for further expansion of that innovation will be provided. However, this funding must be used to translate an innovative product for the benefit of the addiction and recovery patient community.
General
Will we be required to give an interview at any stage of the assessment? If so will this take place in person?
There is no interview requirement at Stage 1 but if you are successful at Stage 1, you will be invited to interview at the Independent Committee meeting for Stage 2 in December. This will take place online.
Is there any weighting assigned to the assessment criteria listed in the supporting information?
The application is assessed in its entirety against the assessment criteria in the Supporting Information for applicants; there is no specific weighting allocated to each criterion.
Do applications require a specific referencing style?
Please use the Vancouver or Harvard style format.
Can one organisation submit two different applications to the same AMI funding call?
Yes, but you would have to clearly indicate the differences, and where applicable, potential synergies. Overlap for activities and costs would not be allowed.
The application form indicates that there is potential for Joint Lead Applicants. Please clarify the implications of this and under what circumstances it would be acceptable.
An application might include Joint Lead Applicants for a number of reasons and is project dependent. Common examples are (but not limited to): if the intended Lead does not have experience of leading large projects; or if there are two co-applicants equally involved in the project from two different organisations. Justification for Joint-Lead applicants must be given. Please note only one organisation (the lead organisation) will be party to the funding contract with DHSC.
Can an application to AMI be submitted whilst waiting for an outcome from another award/funder?
Yes, but this must be explained in the application form. If the project is the same or very similar, applicants will not be awarded funding for both projects.
What evidence is required to demonstrate commitment from a collaborating party at Stage 1 of an application?
You can provide a letter of support, however that is not a requirement at Stage 1. We advise that you make clear in the application what role and responsibilities the collaborators will contribute.
How many co-applicants or collaborators can be involved in the project? And how would the budget be separated between them? Under what criteria should we choose the lead applicant?
Up to 7 co-applicants can be involved in the project (named on your applications) but you may have as many collaborators (not named on your application) as you wish. Each of these must be justified and add value to your work and you would need to ensure that effective control of the project can be maintained. We expect a single collaboration agreement with all the co-applicants and collaborators to ensure this, particularly to protect what happens to intellectual property and the participants' freedom to operate. The budget should be attributed to each collaborator based on the lead applicant cost allocation based on the proposed work.
Can I propose co-applicant organisations be of the same type e.g. two universities?
Yes, co-applicant and collaborating organisations can be of the same type, as long as they are from the list of our eligible organisations. If support for a clinical trial is requested, one of the co-applicant/collaborators must be an NHS organisation.
How long after the outcome of the submitted application is announced is the funding available for use?
The announcement of the outcome of the awards will take place in January 2024 and projects must then start between 26 February 2024 and 11 March 2024. Time to start can vary based on the project depending on the complexity of the queries, but cannot extend outside of these dates.
Do contracts (for example a data sharing agreement with a co-applicant/collaborating NHS organisation) need to be in place before an application is made or can this be completed when/if the proposal is awarded?
There is no requirement for any contracts to be in place prior to applying for AMI. We would expect initial discussions with collaborating co-applicant/collaborators and data access relevant to the project to have started and to reflect their commitment in the application. We request copies of the draft collaboration agreement and any other agreements relevant to the project as part of our due diligence process. Where prior agreements are already in place with co-applicant/collaborators, this should be explained in the proposal and provided during due diligence.
Are AMI Applications confidential?
AMI applications are confidential. More information can be found in our document Confidentiality and disclosure - a guide for applicants, reviewers and commissioning committees. If funded, the Plain English Summary, Chief Investigator/Co Investigator(s) names, award amount, start date and end date will be published on the NIHR funding and awards page.
Can Devolved Nations and organisations based abroad be involved?
The Lead Organisation must be based in the UK. Collaborators based in devolved nations or abroad are allowed. All proposals must demonstrate clear patient benefit to the NHS or broader UK-wide Health System.
Are you able to share a previously successful example application (appropriately redacted)? Where can applicants seek support with application writing?
You may refer to the NIHR funding and awards page for examples of funded projects. Unfortunately, we are not able to share a previously successful example application. We would advise getting in touch with your local NIHR Research Design Service (RDS). The RDS provides support to health and social care researchers across England on all aspects of developing and writing a grant application including research design, research methods, identifying funding sources and involving patients and the public. Advice is confidential and free of charge.
Is it possible for an application to change between Stage 1 and Stage 2, and if so, to what extent?
The overall aim of the project shouldn't change but it is possible to add or remove work packages if justified. In this situation, applicants would be advised to discuss the changes in advance of the submission of their Stage 2 application with the AMI Programme Team. This also applies to budget changes over 10% from Stage 1.
Finance and Intellectual Property (IP)
How are the funds paid out? What is the percentage of total costs budgeted usually granted on average? How are funds distributed to co-applicants/collaborators?
AMI provides funding for 100% of research costs, normally paid quarterly in arrears according to a payment schedule defined at the contracting stage. For HEIs, up to 80% of Full Economic Cost will be paid, provided that TRAC methodology has been used. For applications where the contractor is an NHS body or a commercial organisation, up to 100% of costs will be paid. For more information see the i4i Finance guidance.
Payments will be made to the contracted organisation only and the contracted organisation will be responsible for passing on any money due to their co-applicant/collaborator organisation(s). Appropriate sub-contracts must be put in place for any element of the research which is to be paid to another organisation.
Can early work conducted now, before funding is awarded, still be claimed and funded at a later date if successful, or are there restrictions on when costs are incurred i.e. this must be after the project start date?
Earlier work, prior to project commencement will not be funded and therefore should not be claimed. Project costs can only be incurred from the start date of the project.
What level of finance detail is required at Stage 1 application?
The estimated research cost and estimated NHS support and treatment costs must be included within the application form at Stage 1. Stage 2 requires a detailed breakdown of these costs.
Where can I find more information on what to include as NHS support and treatment costs?
Local Clinical Research Networks are there to advise on NHS support and treatment costs. A summary of what these costs include can be found in the i4i finance guidance.
Are University staff costs funded, such as a PostDoc salary or time?
The appropriate amount of a PostDoc salary for their time contribution to the project is eligible. The Awards are for projects, so costs are looked at on that basis.
Is there a limit to subcontracting fees for e.g. IP/FTO searches and patent filings throughout the project?
There is no limit to the amount paid to subcontractors. However, these costs need to be fully justified. Please note that one of the criteria for assessing applications is whether the project would provide good value for money and subcontracting costs may be considered as part of this.
Can the cost of capital equipment be included in the grant application?
Capital equipment costing up to £5k, excluding VAT, will be considered. Costs of computers are normally restricted to a maximum of £650 each excluding VAT. For a more detailed overview please refer to the Finance Guidance.
Can rental of a piece of capital equipment be included?
Rental for a piece of capital equipment can be included within the application, but the need to do so must be justified.
Can I claim placebo manufacture within the project costs?
Yes, as long as it is part of the study/trial, appropriate placebo manufacturing costs are eligible for the funding.
What happens with arising IP if it is an improvement to existing IP owned by an industry/commercial co-applicant/collaborator?
Contracting organisations must disclose any encumbered background IP which is required for the purpose of the NIHR funded project. As with any NIHR funded foreground IP generated in the project, the ownership of improvement to any background IP will need to be agreed upon during the contracting stages.
Does the applicant need to have secured a patent before applying? How can the applicant demonstrate the IP value of his proposal?
There is no requirement for having a patent granted at the time of submission of an application. However, we are looking for strong evidence that the level of innovation in the proposed technology is high. Providing competitor analysis, the level of innovativeness and clearly describing how the proposed technology stands out from any similar ones or any alternative solutions to the same problem would be taken into account. Seeking a professional consultation with a patent attorney could be an additional way of supporting the strong IP position of the project further.
Can you say more about establishing Freedom to Operate? What evidence is needed for this?
The ideal evidence for clear FTO would be provided by a professional search conducted by an IP or Patent law firm. However, this is not a requirement for submitting an application for the programme and internal FTO searches are acceptable at this stage.

