
Apply to register a non-commercial study onto the Associate PI Scheme

Register your NIHR Portfolio research study for the Associate PI Scheme.
Health and care professionals are mentored by a local PI and can help deliver trials.
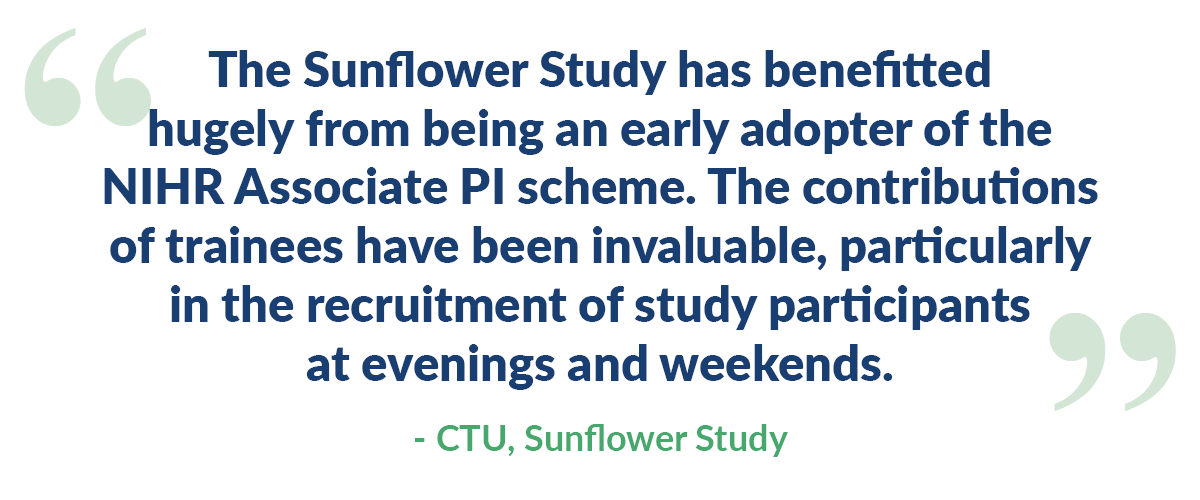
Study teams have told us that having Associate PIs work on their studies:
If the statements below apply to your study, then the Associate PI Scheme could be the ideal opportunity for your research. My study:
Please read see our FAQs for for more full details of Associate PI Scheme eligibility criteria.
Before you get started, we highly recommend you watch the study application instructions video below.
For a study to be eligible for the Associate PI Scheme, it must meet the eligibility criteria that is laid out in our study eligibility criteria. If you have further questions about the eligibility of your study, please contact the Associate PI Scheme central team at associatepischeme@nihr.ac.uk.
It is the responsibility of the study team to register the study for the Associate PI Scheme. Studies can be registered by a member of the Study Team, such as the Trial Manager or Chief Investigator, or the Director/Deputy Director of the Clinical Trials Unit.
Once we receive your application, it will be assessed by the Associate PI Scheme Team against the eligibility criteria. Confirmation will also be sought from the CI of the study and the nominated signatory from the study’s Managing Organisation.
Application Tip: Some nhs.uk & nhs.net accounts may experience issues receiving our emails. Please check your 'Junk/Spam' email folder and mark the associatepischeme@nihr.ac.uk as 'not Junk' or 'safe' to avoid future issues. Thank you.
Once we have assessed your study is eligible, you will receive an email confirming that your study has joined the Associate PI Scheme, and that applicants are now able to apply to contribute towards your study on the Associate PI Scheme.
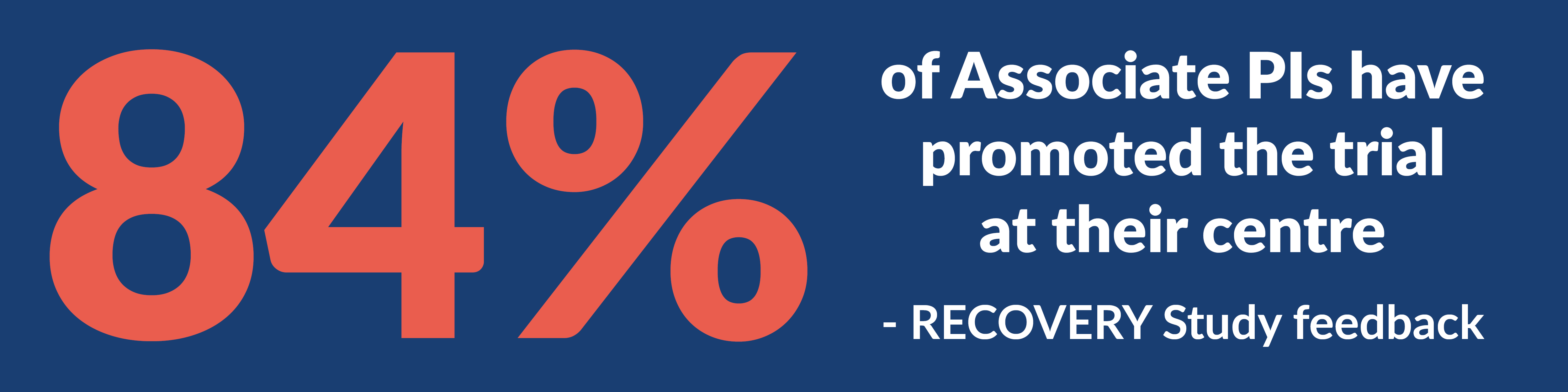
We ask that you make all of your sites aware that your study is on the Associate PI Scheme so that your sites and local PIs can encourage individuals to join the scheme.
To support this we have collated useful resources that provide guidance, suggested prompts, and promotional materials that will hopefully ensure your study and Associate PI trainees gain the most out of involvement in the scheme.
Visit the Associate PI Scheme resources website
The study coordinator for your study will be given access to a dashboard that allows them to view who all of the confirmed Associate PI trainees are for their study(ies), so that they can make contact with them and encourage interaction with the National Study Team. The dashboard also details Associate PIs who have completed the scheme and are now fully certified.
Full details of how to access the Dashboard will be sent to the study coordinator.
We encourage that certified Associate PIs are acknowledged in any publications from the study in a PubMed searchable way, such as in the appendix, as a collaborator.
If you have any questions, including about the eligibility of a study to join the scheme, please contact the Associate PI Scheme central team at associatepischeme@nihr.ac.uk.
You may find the answers to some of your questions in our Associate PI Scheme FAQs.
Need a little extra help? Based on Associate PI trainee feedback, we have created useful resources that provide guidance, suggested prompts, and promotional materials that will hopefully ensure your study and Associate PI trainees gain the most out of involvement in the scheme.