
Published: 16 October 2020
In May, the NIHR published a Framework to support the restarting of research paused due to COVID-19. Developed in partnership with multiple stakeholders and the devolved nations, the framework provides a flexible structure for local decision-making. Our goal is to restore a fully active portfolio of NIHR research while continuing to support important COVID-19 studies as part of the Government response to the pandemic.
Second wave guidance
Earlier this week, the NIHR issued guidance to protect both COVID-19 and non-COVID-19 research during a ‘second wave’ of high COVID-19 activity. The guidance makes very clear that research staff funded by NIHR should not be deployed to front line duties except in exceptional circumstances. This is in contrast to the ‘first wave’ when numerous staff were deployed to the clinical front line. The guidance - which applies to England but was developed in consultation with the devolved nations - has been endorsed by Dr Alison Austin, Deputy Director of Research at NHS England.
Dr William van't Hoff, CEO of the NIHR Clinical Research Network (CRN) and Senior Responsible Officer for the Restart Programme, said: “As the number of people with COVID-19 starts to rise again, it is imperative that our research staff focus on priority COVID-19 studies including vaccine studies. But we also need our teams to continue to support the restart of non-COVID-19 research, both commercial and non-commercial, in tandem with the restoration of NHS routine clinical services. The impact of research being paused in the Spring has been severe and we will make every effort to maintain recruitment rates across the portfolio.”
The guidance stipulates that clinical academic staff should only be deployed within the guidelines recently issued by a working group convened by the UK Clinical Academic Training Forum and the Conference of Postgraduate Medical Deans of the UK. These relate to people in both medical (medical guidance) and non-medical (non-medical guidance) roles.
The latest CRN data
CRN has continued to improve the data summarising the restart of studies* and is now able to share information not just at study level but also for the contributing study-sites. Since these data were first collated in June, soon after the Restart Framework was launched, we have seen a steady and progressive improvement in the number of studies and study-sites that are now open.
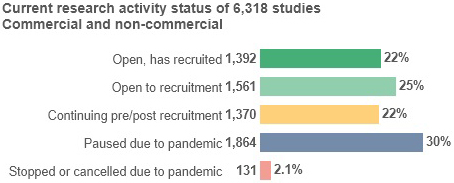
Study information as of 14 October 2020
- Based on data from 6,318 commercial and non-commercial studies, 2,953 studies (47%) are open to recruitment - an increase of 231 studies (2%) since last month - of which 1,392 have recruited (47%).
- A further 1,370 studies (22%) are in setup or follow-up stage of activity and proceeding as planned, up from 1,053 (17%) last month.
- 1,864 studies (30%) are fully paused due to the pandemic, down from 2,176 (36%) last month, and only 131 studies (2%) have been stopped or cancelled entirely.
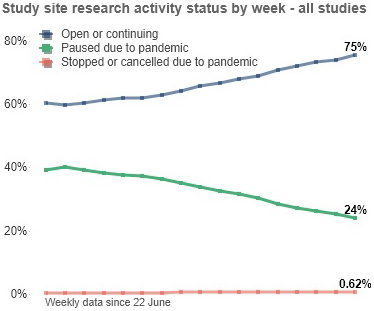
Study site information as of 14 October 2020
- 75% of study sites are open or proceeding as normal, which has improved from 71% last month and from 60% since 22 June.
Funding contract variations and study resilience
The NIHR Research Programmes are also progressing well and many studies are recruiting, although there are some for which the research setting or methodology remains highly problematic - for example studies in schools or workplaces.
A number of revised research plans and protocols have been approved and formal variation to contracts have been requested. For example, the Research for Patient Benefit Programme has received 49 contract variation requests of which 21 have already been approved. A further 24 are anticipated, representing in aggregate about 40% of the active portfolio. In the case of the Health Technology Assessment programme, 46 variations have been approved in the first two quarters of this financial year, which is 10% of the current live portfolio. These are mainly for additional time.
In response to the changing profile of NHS services and patient pathways and to the demands of the nationally prioritised COVID-19 studies, researchers have been amending study protocols to add resilience so they can successfully continue to recruit and make sure findings remain relevant. Learning captured from across this active portfolio will build study resilience and help to future proof the design of new studies, which it is hoped will limit the effect of future outbreaks.
Working with partner organisations
Over the past few months the NIHR CRN have been working very closely with NHS R&D. William van’t Hoff was recently invited to meet with UKRD and R&D Forum leadership to discuss Restart as well as future opportunities for collaboration. Operationally, weekly contact via the Forum has allowed for sharing of best practices, supporting staff and collaboration on processes and practices.
Back in September, Christine McGrath, UKRD Chair, and Kate Greenwood, R&D Forum Manager, were invited to the NIHR CRN Industry Roadmap meeting also attended by ABPI and representatives from the life sciences industry. Sharon Barrett, Head of Research Operations at CRN, said: “This was the first time NIHR, NHS R&D and industry have come together to discuss Restart at an operational level. The strongest takeaway message was that only by working together and communicating regularly can we build on the lessons learned during the pandemic and restore a balanced portfolio of clinical trials.”
The Restart Advisory Group comprises 23 members of key health research organisations and is co-chaired by Sheuli Porkess, Executive Director Research Medical and Innovation at the ABPI and Aisling Burnand MBE, CEO of the AMRC. The group, key to highlighting major issues and opportunities for restarting research, recently submitted a list of priority actions for NIHR and NHS England. This has helped to reinforce the vital relationship between NIHR and NHSE, which has this month led to a series of meetings and further collaborations to drive Restart.
*This includes any study taking place in the UK listed on NIHR CRN’s Central Portfolio Management System which is currently open, in setup, paused, or closed since the start of the COVID-19 crisis (18 March 2020).
Please note that these data include clinical trials of investigational medicinal products (CTIMPs) and other clinical research (e.g. non-CTIMP interventional or observational studies).
If you have a question that is not covered in this update or listed in the Q&A on the impact of COVID-19 on research funded or supported by NIHR please use the contact details at the bottom of this statement.
Further resources are available on the 'Restarting research' page, including links to previous updates, examples of best practice and resources to help reassure patients that it's safe to get involved in research, like this short animation.

