Executive summary
Prospective trial registration is critical to the transparency of clinical trials funded by NIHR. The NIHR policy on clinical trials aims to improve practice in prospective registration, with the objective of raising the probability of impact of NIHR-funded research. An audit of clinical trials in scope (N=351, between 20 May 2019 and 19 May 2020) showed that 329 clinical trials (94%) had notified the NIHR of their registration in a public registry. Further analysis of the NIHR clinical trials registered in the ISRCTN registry (N=-298) highlighted that 230 of those (77%) had complied with the NIHR’s requirement to register prospectively.
Key learning from the audit recognised that interoperability and ongoing cross centre validation of datasets are imperatives for any future audit. Areas for improvement across the NIHR include the need for systematic practices, alongside maximising current processes to support the collection and monitoring of trial registration information. Future work will also benefit from collaboration with our partners, including the HRA and public registries.
Background
The NIHR adopted its policy on clinical trial registration and disclosure of results on 20 May 2019, in response to signing the WHO Joint Statement.
Prospective trial registration and timely disclosure of results are critical to ensure full transparency of clinical trials funded by the NIHR. These principles are also important for ethical, moral, accountability, research integrity and waste reduction perspectives. They also support the Health Research Authority drive for greater research transparency, through its Make it Public strategy.
The policy aims to improve practice in prospective registration and timely disclosure of results, with the objective of raising the probability of impact of NIHR-funded research through greater transparency. This in turn, supports the NIHR’s mission to improve the health and wealth of the nation through research.
Objective and measures
The objective of this audit was to evaluate registration compliance of fully funded NIHR clinical trials (as defined by AcoRD) recruiting between 20 May 2019 and 19 May 2020, and subsequently identify areas for improvement.
The primary measure of compliance was the proportion of all NIHR-funded clinical trials that were registered in a clinical trial registry. A secondary measure of clinical trials registered in the ISRCTN public registry before the start of recruitment was used as an indicator of prospective trial registration compliance. The audit was conducted using NIHR systems and other automated data retrieval, to reduce burden and manual handling of data. This audit therefore effectively reports where the NIHR has been provided trial registration details and it is possible a clinical trial has been registered, but not notified to the NIHR. It should be noted that although the HEE/NIHR Integrated Clinical Academic Programme is not funded by the NIHR, the programme is managed and delivered by the NIHR, and adheres to NIHR policy and processes, and were therefore included for completeness. However, it should be noted that a definition of infrastructure projects funded through the NIHR, which would fall within the remit of a future award, is in the process of being agreed.
Results
A baseline of 540 studies were identified as clinical trials within the NIHR Clinical Trials Overview (CTO) and the Clinical Research Network (CRN) databases between 20 May 2019 and 19 May 2020.
351 of these awards were actively recruiting and therefore in scope, of which 329 (93.73%) had notified the NIHR of their registration in a public trial registry.
Of these 329 studies, further analysis was undertaken using data from the ISRCTN public registry, which represents the majority of NIHR registrations (see Table 1), to identify the proportion of trials that registered prospectively e.g. before recruitment started. 298 of the 351 clinical trials in scope were identified in the ISRCTN database (see Table 1), of this 298, 233 were solely registered in ISRCTN, 14 were registered in ISRCTN and CTGov, 46 were registered in ISRCTN and EUDRACT and 5 were registered in ISRCTN, EUDRACT and CTGov. These clinical trials with multiple registrations were from the EME, HTA, IAT and RfPB (see Table 1). Of the 298 studies registered in ISRCTN, 230 (77.2%) clinical trials had complied with the NIHR’s prospective requirements, having been registered prior to starting recruitment, whereas 68 (22.8%) were registered after starting recruitment. It was not possible to retrieve registration details from these additional public registries at this stage, including EudraCT and clinicaltrials.gov.
Table 1 - Trial Registry - Registration by NIHR programme: Table 1 shows trial registration by registries for each of the 10 programmes.
|
Registries |
EME |
HS&DR |
HTA |
IAT |
I4I |
F'ships |
P'ships |
PGfAR |
PHR |
RFPB |
Grand Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
CTGov |
1 |
- |
1 |
2 |
- |
3 |
2 |
1 |
2 |
14 |
26 |
|
EUDRACT |
- |
- |
1 |
- |
- |
1 |
- |
- |
- |
2 |
4 |
|
ISRCTN |
17 |
3 |
104 |
9 |
2 |
20 |
2 |
21 |
8 |
47 |
233 |
|
ISRCTN/CTGov |
1 |
- |
9 |
2 |
- |
- |
- |
- |
- |
2 |
14 |
|
ISRCTN/EUDRACT |
16 |
- |
20 |
- |
- |
- |
- |
- |
- |
10 |
46 |
|
ISRCTN/EUDRACT/CTGov |
3 |
- |
2 |
- |
- |
- |
- |
- |
- |
- |
5 |
|
NO REG FOUND |
- |
- |
- |
10 |
- |
12 |
- |
- |
- |
- |
22 |
|
UNKNOWN REG |
- |
- |
- |
- |
- |
- |
- |
1 |
- |
- |
1 |
|
Grand Total |
38 |
3 |
137 |
23 |
2 |
36 |
4 |
23 |
10 |
75 |
351 |
Registration compliance was highest for interventional studies, Government/NHS funded trials and projects funded through commissioned calls, whereas compliance was lowest for observational studies, trainee development and academic work streams, however the latter was only negligibly lower. Early indications suggest that lower registration rates may be linked to a lack of awareness of NIHR requirements and limited monitoring practices for these programmes. More detailed information on registration per programme, research type, study design, programme stream and organisation type are detailed in Appendix 1.
Appendix 2 provides the list of institutions with clinical trials in scope and recruiting within the audit period from 20 May 2019 to 19 May 2020, alongside the number of registrations notified to the NIHR, as outlined in the NIHR policy.
Comparison to other audits
In 2017, the NIHR conducted an early review of the proportion of primary and secondary research studies which had registered. A sample of trials (N=175) across all NIHR domestic programmes in 2017 identified that 79% of the trials selected had registered (N=138). The latest NIHR audit highlighting a 94% compliance rate, therefore shows considerable improvement since the NIHR signed up to the WHO Joint Statement in May 2018.
More widely, the MRC Review of Clinical Trials (2018) identified that of the 175 clinical trials they funded, 100% were registered, with 55% on ISRCTN, this marked an increase from 94% in 2017.
In the HRA Clinical Trial Registration Audit after excluding those with valid deferrals, there were 599 studies, of which 405 (67.61%) were registered. Of the 405 registered studies, 58 were from phase 1 clinical trials, 138 from clinical trials of medical devices and 209 from other clinical trials. Of the 194 studies not registered, 9 were from phase 1 clinical trials, 67 from clinical trials of medical devices and 118 from other clinical trials. This means the compliance for registration of phase 1 clinical trials was 86.5%, 67.3% for clinical trials of medical devices and 63.9% for other clinical trials.
The NIHR data shows similarities to the MRC 2017 data, albeit with higher study numbers and different study types which may explain the lower registration rate compared to the MRC. Overall, the NIHR registration compliance is significantly higher than the HRA cross sector audit. As evidenced in the HRA’s audit phase 1 clinical trials had the highest rate of registration, and similarly the NIHR data showed higher compliance with registration for interventional studies compared to observational studies. Furthermore, the registration audit of clinical trials given a favourable opinion by UK research ethics committees, reported an 80% compliance with registration for the 1014 clinical trials in the report.
Data analysis and lessons learnt
The extraction and analysis of available related data sources for the purposes of this audit, required considerable collaboration and business intelligence expertise across NIHR Centres. The key principle was to minimise burden on the NIHR and award holders, avoiding the need for project teams to provide and respond to data requests, particularly as this audit was conducted during the height of the COVID-19 pandemic. However, lessons learnt during this audit should support the intention that the future reporting of compliance of any NIHR policy should be more efficient and quality assured.
In assessing the range of system data available to identify NIHR clinical trials in scope as defined by the related NIHR policy, the most related data source was the NIHR Clinical Trials Overview (CTO) database, which is compiled and verified 6-monthly across all NIHR awards. The CTO data set was subsequently validated against the Clinical Research Network’s (CRN) data management system, which identified some potential data discrepancies. 81 projects were included in the CTO data set but not the CRN data set. These were largely where recruitment had not yet started and therefore these trials were excluded as they were not in scope. However, there were other data gaps which need to be reviewed and validated going forward. There were 85 awards included in the CRN dataset that were not in the CTO dataset. These were primarily observational studies, which were considered out of scope for this audit.
NIHR reporting is currently conducted at award or programme level, however, this has considerable limitations as a large proportion of NIHR projects include more than one study. This is particularly an issue for larger multiple project awards where registration needs to be monitored at multiple levels. The principle of presenting one award with a number of associated projects (or trials) remains an issue that needs to be resolved across all NIHR studies. Resolution of this will improve data intelligence and reporting mechanisms to report at study level. A further consideration is that each study of any project may be registered at the appropriate time point, but this detail is not currently available through automated systems at this stage.
The NIHR systems’ data sets provide a rich range of valuable intelligence on each project, including project start date, recruitment, methodology, institution type etc. However, to fully validate compliance with the NIHR clinical trial policy further validation was required to identify the date of registration. Therefore, an automated download for the key trial registries including ISRCTN, Eudract and clinicaltirals.gov was required. For the purposes of this audit, however, data was only available from the ISRCTN database. Further work is needed to ensure this data is systematically available across the NIHR, with the intention of adding the registration data to the NIHR Open data set for the 2021 audit. Continuing to work with NIHR delivery partners, including trial registries and the Health Research Authority’s implementation of its ‘Make it Public’ transparency strategy, remains critical to a coordinated whole system response.
The NIHR is not currently able to report full compliance with its clinical trial policy using automated data retrieval and reporting, due to inconsistent reporting and monitoring across programmes and institutions types. Initial discussions suggest that compliance is stronger where requirements are clearly advised, monitored and practice is embedded, and compliance routinely monitored. It remains important to embed and promote awareness to realise changes in practice for prospective registration to ensure greater clinical trial transparency. It was also noted that the CRN offers support to register their studies in ISRCTN, and opportunities to optimise and promote this support should be highlighted where relevant to project teams. However, it should be noted that delivery of this policy presents some tensions against the current drivers for busting bureaucracy.
Finally, the capacity of the NIHR and contracting organisations to support behaviour change and ensure compliance of trial registration within their sphere of influence remains paramount. The CRN support to register trials is not mandatory and not fully utilised, yet it provides a strong example of the potential value and reduced burden that can be achieved. Where possible embedded and automated processes should support this to minimise burden Contracting institutions are also in a strong position to support their studies to ensure compliance in a more efficient and effective way.
Emerging recommendations for improving automated reporting of clinical trial registration include:
- Improvement of interoperability and connectivity across NIHR data management systems
- Ensuring the completeness of clinical trials data across the full range of NIHR data sources, with the registration data integrated into the NIHR Open dataset for the 2021 audit
- Deliver greater accuracy and consistency of data and definitions of what is in scope, including regular cleansing and validation
- Develop mechanisms to improve registration reporting beyond programmes/awards to study level across the NIHR
- Systematic cross NIHR monitoring of compliance against the policy
- Raising awareness of compliance amongst researchers and contracting organisations and promoting existing support mechanisms e.g. CRN support to register a trial in ISRCTN
- Working with key partners and stakeholders e.g. HRA, trial registries, to deliver a whole system approach to good clinical trial practice to maximise automation and minimize duplication
- Maintaining automated and up to date information through access to publicly available trials registration data
Future development and next steps
There was a significant level of learning about the potential intelligence and value of NIHR systems data. This audit demonstrated that overall, the NIHR systematically ensures and records registration of its funded awards. However, as highlighted above there remains some clear actions that can be taken to improve the efficiency, effectiveness, and quality of reporting against this policy. In addition. Some longer-term issues should be considered to ensure wider understanding of the level and detail of compliance against this policy e.g. whether trial protocols are published, registries are updated, summary results published etc.
Wider NIHR learning and future activity that is expected to have an impact on future compliance against this policy includes:
- Ensuring all the NIHR policy requirements are embedded in programme monitoring practices
- Identifying reporting mechanisms for the future audit of compliance against the wider requirements of the NIHR Clinical Trial Policy, on the reporting of summary results and publication of trial findings.
- Greater alignment and consideration of the implication of this policy with the drive for busting bureaucracy
- Working closely with the HRA on the delivery of the Make it Public Strategy
- Aligning NIHR policy implementation timelines to ensure key project documentation e.g. data management and access plans, dissemination plans etc are available from the outset of the project and updated in a timely manner
Assumptions and limitations of the data
In keeping with the policy’s principles of minimising burden, maximising the use of systems and data, whilst mindful that retrospective compliance is not mandatory, this audit is only reporting against NIHR awards in scope i.e. clinical trials where all of the research costs are funded by the NIHR (however, this included those jointly funded by the NIHR/MRC and NIHR Academy awards funded by Health Education England [HEE] and recruiting between 20 May 2019 and 19 May 2020.
One year on from the policy’s launch, it is also acknowledged that none of the trials will have reached 12 months after primary study completion, when summary results should be published. Therefore, for clarity of purpose, this first audit limited its focus on compliance against the NIHR’s requirement to register the clinical trial before the first participant receives an intervention. However, as this information was difficult to obtain through automated means a proxy measure of recruitment start date was used to assess compliance.
Data quality
Data quality checks for the audit were limited to the checking for data gaps or irregularities by the individual NIHR centre, with some additional spot checks on registration IDs.
It was intended that the data sets would be cross-checked against the NIHR-preferred trial registries including ISRCTN, EuDract, and Clinicaltrials.gov. However, this was only conducted against the ISRCTN database, as the data could not be retrieved from the other registries at this time.
The data used for this audit was sourced from a combination of the NIHR Clinical Trials Overview dataset (updated every 6 months) and the Clinical Research Network data management system (updated daily). The CTO dataset was last updated in May 2020 (over a four week submission period) forming a comprehensive cross-NIHR dataset.
No validation was completed directly with researchers or PIs to minimise burden, and the data quality relies on the notification of registration details. However, it is recognised that this may lead to shortfalls in the data and missed opportunity of raising awareness of the policy. The results show relatively strong awareness of researchers, supported by programme monitoring and additional support to register by CRN, although there is clearly some work to raise awareness of requirements amongst some key research groups and award types.
Conclusion
As a signatory to the WHO Joint Statement, the NIHR has committed to monitor compliance of NIHR studies in line with the NIHR Policy for Clinical Trials Registration and Disclosure of Results. The audit found, using automated available data sources, that the majority (90%) of clinical trials were registered with ISRCTN, the NIHR preferred registry, with 65 (19.9%) registered more than once which is not an NIHR requirement and potentially adding undue burden on research teams. However, the requirement to register the trial largely remains a task for researchers, and where possible collaboration, support and automation should remain the focus for future development in this area of compliance.
Additional Registration Audit Data
Trials by Registry
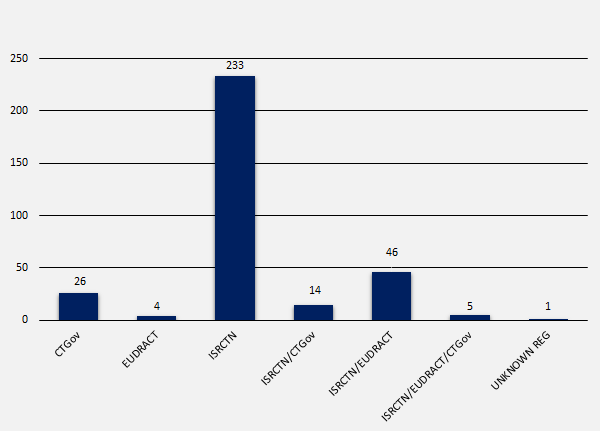
NIHR trials were registered with six different registries and one unknown registry. In total, 329 trials were registered with most registrations (71%) with the ISRCTN (International Standard Randomised Controlled Trial Number), and the least registrations (1%) with the EUDRACT (European Union Drug Regulating Authorities Clinical Trials Database). The number of registrations per registry were as follows:
- CTGov (ClinicalTrials.gov) = 26 (7.9%)
- EUDRACT = 4 (1.2%)
- ISRCTN = 233 (70.8%)
- ISRCTN and CTGov = 14 (4.3%)
- ISRCTN and EUDRACT = 46 (14%)
- ISRCTN, EUDRACT and CTGov = 5 (1.5%)
- Unknown registry = 1 (0.3%)
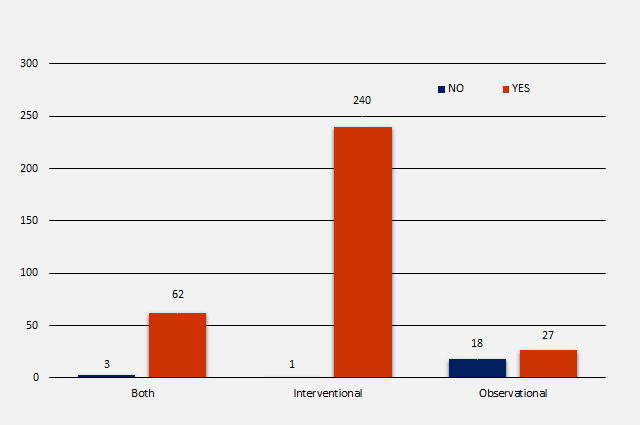
Trial Registration by Study Design
In total there were 351 NIHR trials identified in scope, of which 45 (12.8%) were observational, 241 (68.7%) were interventional and 65 (18.5%) were of both designs. The number of registrations per study design were as follows:
- 45 observational studies = 18 (40%) not registered and 27 (60%) registered
- 241 interventional studies = 1 (0.4%) not registered and 240 (99.6%) registered
- 65 of both designs = 3 (4.6%) not registered and 62 (95.4%) registered
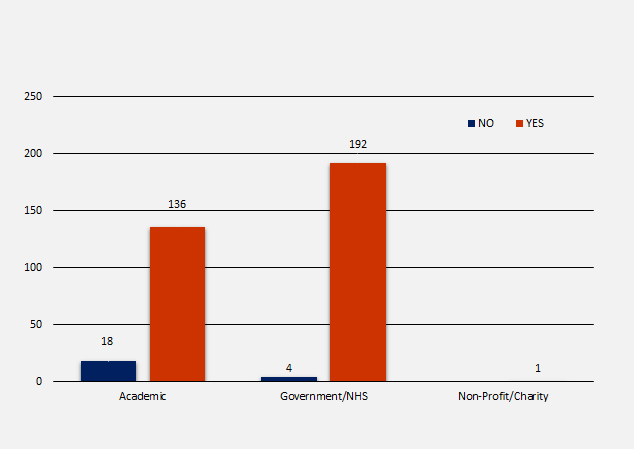
Trial Registration by Contractor Organisation Type
There are three contractor categories relating to clinical trials funded by the NIHR; academic, government/NHS and non-profit/charity. In total there were 351 NIHR trials identified in scope, of which 154 (43.9%) were academic organisations, 196 (55.8%) were government/NHS organisations and 1 (0.3%) was non-profit/charity. The number of registrations per organisation type were as follows:
- 154 academic = 18 (11.7%) not registered and 136 (88.3%) registered
- 196 government/NHS = 4 (2%) not registered and 192 (98%) registered
- 1 non-profit/charity = 1 (100%) registered
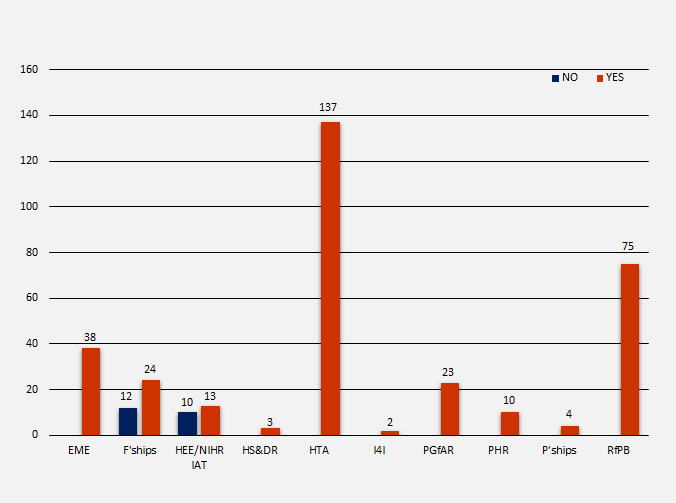
Trial Registration by NIHR Programme
There are ten NIHR funding programmes and awards. Of the 351 NIHR trials identified in scope (329 registered [93.7%] and 22 not registered [6.3%]), the funding was allocated as follows: 38 (10.8%) for Efficacy and Mechanism Evaluation (EME), 36 (10.3%) for NIHR Fellowships (F’ships), 23 (6.6%) for Health Education England/NIHR Integrated Clinical Academic Programme (HEE/NIHR IAT), 3 (0.9%) for Health Services and Delivery Research (HS&DR), 137 (39%) for Health Technology Assessment (HTA), 2 (0.6%) for Invention for Innovation (I4I), 23 (6.6%) for Programme Grants for Applied Research (PGfAR), 10 (2.8%) for Public Health Research (PHR), 4 (1%) for NIHR Professorships (P’ships) and 75 (21.4%) for Research for Patient Benefit (RfPB). The number of registrations per programme were as follows:
- 38 EME = 38 (100%) registered
- 36 F’ships = 12 (33.3%) not registered and 24 (66.7%) registered
- 23 HEE/NIHR IAT = 10 (43.5%) not registered and 13 (56.5%) registered
- 3 HS&DR = 3 (100%) registered
- 137 HTA = 137 (100%) registered
- 2 I4I = 2 (100%) registered
- 23 PGfAR = 23 (100%) registered
- 10 PHR = 10 (100%) registered
- 4 P’ships = 4 (100%) registered75 RfPB = 75 (100%) registered
Trial Registration by Funding Stream
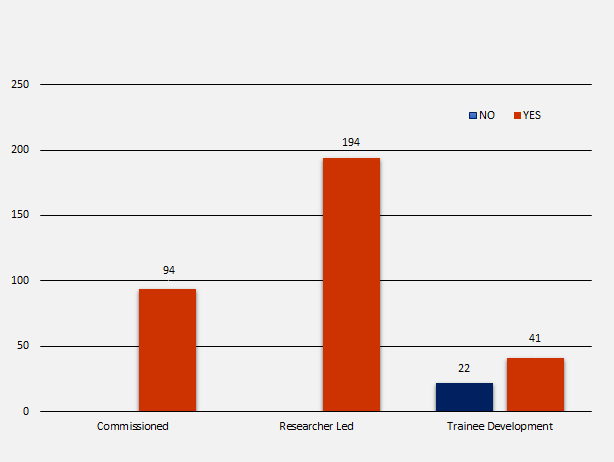
There are three NIHR funding streams which are commissioned, researcher-led and trainee development. In total there were 351 NIHR trials identified in scope, of which 94 (26.8%) were commissioned trials, 194 (55.3%) were researcher-led and 63 (17.9%) were for trainee development. The number of registrations per programme stream were as follows:
- 94 commissioned = 94 (100%) registered
- 194 researcher-led = 194 (100%) registered
- 63 trainee development = 22 (34.9%) not registered and 41 (65.1%) registered
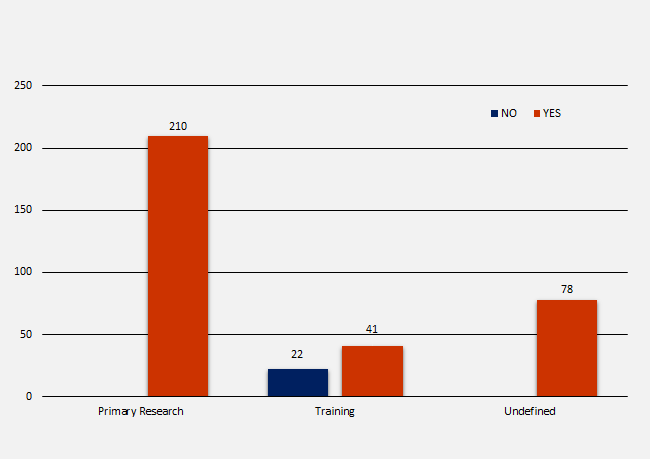
Trial Registration by Research Type
There were three research types listed; primary research, training and undefined. In total there were 351 NIHR trials identified in scope, of which 210 (59.8%) were primary research, 63 (17.9%) were training and 78 (22.2%) were undefined. The number of registrations per research type were as follows:
- 210 primary research = 210 (100%) registered
- 63 training = 22 (34.9%) not registered and 41 (65.1%) registered
- 78 undefined = 78 (100%) registered
Appendix 2
NIHR Clinical Trials by Sponsoring Institution
Of the 351 NIHR trials identified as in scope, 329 (93.7%) were registered and 22 (6.3%) were not registered. The following table lists whether the clinical trials in scope were reported to the NIHR as registered or not, by sponsoring institutions.
|
Sponsoring Institution |
Not Registered |
Registered |
Grand Total |
|---|---|---|---|
|
Aintree University Hospital NHS Foundation Trust |
1 |
1 |
2 |
|
Alder Hey Children's NHS Foundation Trust |
- |
5 |
5 |
|
Avon and Wiltshire Mental Health Partnership NHS Trust |
- |
1 |
1 |
|
Barts Health NHS Trust |
- |
6 |
6 |
|
Berkshire Healthcare NHS Foundation Trust |
- |
1 |
1 |
|
Birmingham Women's NHS Foundation Trust |
- |
1 |
1 |
|
Bradford Teaching Hospitals NHS Foundation Trust |
- |
1 |
1 |
|
Brighton and Sussex University Hospitals NHS Trust |
- |
1 |
1 |
|
Cambridge University Hospitals NHS Foundation Trust |
- |
5 |
5 |
|
Cambridgeshire and Peterborough NHS Foundation Trust |
- |
1 |
1 |
|
Camden and Islington NHS Foundation Trust |
- |
1 |
1 |
|
Cardiff and Vale University Local Health Board |
- |
1 |
1 |
|
Cardiff University |
- |
5 |
5 |
|
Central and North West London NHS Foundation Trust |
- |
2 |
2 |
|
Christie NHS Foundation Trust |
- |
1 |
1 |
|
Common Services Agency |
- |
1 |
1 |
|
Cornwall Partnership NHS Foundation Trust |
- |
1 |
1 |
|
Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust |
- |
2 |
2 |
|
Derby Hospitals NHS Foundation Trust |
- |
1 |
1 |
|
Devon Partnership NHS Trust |
- |
2 |
2 |
|
East London NHS Foundation Trust |
- |
2 |
2 |
|
Glasgow Caledonian University |
- |
1 |
1 |
|
Great Ormond Street Hospital for Children NHS Foundation Trust |
- |
3 |
3 |
|
Greater Manchester Mental Health NHS Foundation Trust |
- |
7 |
7 |
|
Guy's and St Thomas' NHS Foundation Trust |
- |
9 |
9 |
|
Hertfordshire Partnership University NHS Foundation Trust |
- |
1 |
1 |
|
Hull and East Yorkshire Hospitals NHS Trust |
- |
2 |
2 |
|
Hull University Teaching Hospitals NHS Trust |
- |
2 |
2 |
|
Imperial College Healthcare NHS Trust |
- |
1 |
1 |
|
Imperial College London |
- |
7 |
7 |
|
Imperial College of Science, Technology and Medicine |
1 |
3 |
4 |
|
Intensive Care National Audit & Research Centre |
- |
1 |
1 |
|
Keele University |
1 |
1 |
2 |
|
King's College Hospital NHS Foundation Trust |
- |
2 |
2 |
|
King's College London |
4 |
11 |
15 |
|
Lancashire Care NHS Foundation Trust |
- |
2 |
2 |
|
Leeds and York Partnership NHS Foundation Trust |
- |
3 |
3 |
|
Leeds Teaching Hospitals NHS Trust |
- |
5 |
5 |
|
Liverpool University Hospitals NHS Foundation Trust |
- |
1 |
1 |
|
Manchester University NHS Foundation Trust |
- |
4 |
4 |
|
Mid Yorkshire Hospitals NHS Trust |
- |
1 |
1 |
|
Moorfields Eye Hospital NHS Foundation Trust |
- |
2 |
2 |
|
Newcastle University |
- |
2 |
2 |
|
Newcastle Upon Tyne Hospitals NHS Foundation Trust |
- |
4 |
4 |
|
NHS Blood and Transplant |
- |
1 |
1 |
|
NHS Bristol, North Somerset and South Gloucestershire CCG |
- |
7 |
7 |
|
NHS Cambridgeshire and Peterborough CCG |
- |
2 |
2 |
|
NHS Canterbury and Coastal CCG |
- |
1 |
1 |
|
NHS Lambeth CCG |
- |
1 |
1 |
|
NHS North Staffordshire CCG |
- |
4 |
4 |
|
NHS Sheffield CCG |
- |
1 |
1 |
|
NHS South Norfolk CCG |
- |
1 |
1 |
|
Norfolk and Norwich University Hospitals NHS Foundation Trust |
- |
2 |
2 |
|
North Bristol NHS Trust |
- |
4 |
4 |
|
North East London NHS Foundation Trust |
- |
1 |
1 |
|
Northumbria University |
- |
1 |
1 |
|
Nottingham University Hospitals NHS Trust |
- |
4 |
4 |
|
Nottinghamshire Healthcare NHS Foundation Trust |
- |
4 |
4 |
|
Oxford Health NHS Foundation Trust |
- |
2 |
2 |
|
Oxford University Hospitals NHS Foundation Trust |
- |
6 |
6 |
|
Pennine Care NHS Foundation Trust |
1 |
1 |
2 |
|
Queen Mary University of London |
- |
3 |
3 |
|
Queen's University of Belfast |
- |
5 |
5 |
|
Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust |
- |
2 |
2 |
|
Royal Brompton and Harefield NHS Foundation Trust |
- |
7 |
7 |
|
Royal Devon and Exeter NHS Foundation Trust |
- |
2 |
2 |
|
Royal Free London NHS Foundation Trust |
- |
3 |
3 |
|
Royal Liverpool and Broadgreen University Hospitals NHS Trust |
- |
1 |
1 |
|
Royal United Hospitals Bath NHS Foundation Trust |
- |
1 |
1 |
|
Salford Royal NHS Foundation Trust |
- |
2 |
2 |
|
Sandwell and West Birmingham Hospitals NHS Trust |
- |
1 |
1 |
|
Sheffield Health and Social Care NHS Foundation Trust |
- |
1 |
1 |
|
Sheffield Teaching Hospitals NHS Foundation Trust |
- |
3 |
3 |
|
Solent NHS Trust |
- |
1 |
1 |
|
South London and Maudsley NHS Foundation Trust |
- |
1 |
1 |
|
South Tees Hospitals NHS Foundation Trust |
- |
2 |
2 |
|
Southern Health NHS Foundation Trust |
- |
1 |
1 |
|
St George's University Hospitals NHS Foundation Trust |
- |
2 |
2 |
|
St George's, University of London |
- |
1 |
1 |
|
Sussex Partnership NHS Foundation Trust |
- |
3 |
3 |
|
Swansea University |
- |
1 |
1 |
|
Taunton and Somerset NHS Foundation Trust |
- |
1 |
1 |
|
Tees, Esk and Wear Valleys NHS Foundation Trust |
- |
1 |
1 |
|
University College London |
3 |
12 |
15 |
|
University College London Hospitals NHS Foundation Trust |
- |
3 |
3 |
|
University Hospital Southampton NHS Foundation Trust |
1 |
4 |
5 |
|
University Hospitals Birmingham NHS Foundation Trust |
- |
4 |
4 |
|
University Hospitals Bristol NHS Foundation Trust |
- |
3 |
3 |
|
University Hospitals Coventry & Warwickshire NHS Trust |
- |
6 |
6 |
|
University Hospitals of Derby and Burton NHS Foundation Trust |
1 |
2 |
3 |
|
University Hospitals of Leicester NHS Trust |
- |
2 |
2 |
|
University Hospitals Plymouth NHS Trust |
- |
1 |
1 |
|
University of Aberdeen |
- |
6 |
6 |
|
University of Birmingham |
- |
12 |
12 |
|
University of Bristol |
- |
7 |
7 |
|
University of Cambridge |
- |
2 |
2 |
|
University of Central Lancashire |
- |
1 |
1 |
|
University of Dundee |
- |
1 |
1 |
|
University of East Anglia |
1 |
3 |
4 |
|
University of Edinburgh |
- |
5 |
5 |
|
University of Exeter |
1 |
- |
1 |
|
University of Glasgow |
- |
1 |
1 |
|
University of Lancaster |
- |
1 |
1 |
|
University of Leeds |
2 |
5 |
7 |
|
University of Leicester |
1 |
2 |
3 |
|
University of Liverpool |
- |
5 |
5 |
|
University of Manchester |
1 |
1 |
2 |
|
University of Nottingham |
- |
3 |
3 |
|
University of Oxford |
1 |
14 |
15 |
|
University of Plymouth |
1 |
- |
1 |
|
University of Sheffield |
- |
1 |
1 |
|
University of Southampton |
- |
8 |
8 |
|
University of Stirling |
- |
2 |
2 |
|
University of Sussex |
- |
1 |
1 |
|
University of Warwick |
1 |
2 |
3 |
|
Walton Centre NHS Foundation Trust |
- |
1 |
1 |
|
West London Mental Health NHS Trust |
- |
1 |
1 |
|
Whittington Health NHS Trust |
- |
1 |
1 |
|
Yorkshire Ambulance Service NHS Trust |
- |
1 |
1 |
|
Grand Total |
22 |
329 |
351 |