Before you can start an application you will be required to register on the ARAMIS system. You will be asked to supply a valid email address and to complete some basic information. Once this has been submitted you will receive an email confirming your registration and a temporary password. You should follow the instructions in the email to log onto the system.
Once signed into the system you will be able to update various details including your CV (in ‘manage my details’) and apply for any open applications. To start an application you will need to go to ‘My Applications’ and select ‘New Application’. You should then select the level of fellowship you wish to apply for from the list provided.
After answering all the eligibility questions you will be able to start completing the online form. Please make sure you read all available guidance text including this document as well as any online instructions thoroughly whilst you are completing the form.
Section 1: Application Summary Information
1.1 Host Organisation
Please give details of the organisation who will be the contractor if the award is funded.
If the name of your host organisation does not appear in the pre-populated list please email academy-awards@nihr.ac.uk. It can take up to three working days to add an organisation so this should be done as early as possible to avoid any delays in the application process.
Please note that we expect the Awardee’s proposed host organisation (substantive employer) to act as the contractor. The contractor is expected to:
- Respond to quarterly financial reconciliation exercises, provide the final financial reconciliation statement for the award and to provide ad hoc requests for financial information during the lifetime of the award.
- respond to any queries relating to Intellectual Property, commercialisation and benefit realisation.
1.2 Partner Organisation (where applicable)
Please give details of the main partner organisation who will be supporting this application (for which a signatory will be required).
If the name of your main partner organisation does not appear in the pre-populated list please email academy-awards@nihr.ac.uk. It can take up to three working days to add an organisation.
1.3 Other Partner Organisations
You can also list other relevant partner organisations but a signatory is only required for the main partner organisation.
1.4 Research Title
The programme title should state clearly and concisely the proposed research. Any abbreviations should be spelled out in full.
1.5 Research Type
Select the appropriate research type. If you are not sure which category to select, choose the closest match to your project as this can be adjusted later. View NIHR definitions of terms.
1.6 Award Duration
ou may undertake the award on a part time basis between 80% whole time equivalent (WTE) and 100% WTE for personal reasons but this must be discussed with the NIHR before submission. Please contact academy-awards@nihr.ac.uk.
1.7 Proposed Start Date
This should be from the 1st of the month regardless of whether this is a working day or not. Please be realistic about your possible start date taking account of the necessary contracting, and staff recruitment prior to starting your project.
Section 2: Applicant CV
2.1 Degrees and Professional Qualifications
Please give the full details of any completed higher degree(s) and, where relevant, the full details of any higher degree(s) you are currently undertaking.
2.2 Present and previous positions
When entering details of your current and previous positions please indicate at what percentage (WTE) in each post you were undertaking research.
2.3 Research grants held
Details of all grants obtained in the last five years should be provided, including personal research training awards or fellowships, plus any additional previous grants relevant to this application. Please indicate clearly any co-Awardees and provide brief details of the nature and full extent of your involvement (e.g. project design, project management, day to day running, data collection, data analysis, writing papers for publication.).
In the ‘Role in Research Grant’ box for each entry include the registration number and name of the registry and the DOI of the main related publication. Where the study is still ongoing or final results have not yet been published, please provide an estimated publication date.
This is inline with the NIHR policy on clinical trial registration and disclosure of results. These details relate to the applicant only. Information on the grants/awards held by the nominating institutions is no longer required.
Please note that your research grant record must be completed within the application form and not via the CV section on ARAMIS.
2.4 Relevant Prizes, Awards and Other Academic Distinctions
Please provide details of any awards or distinctions that would be relevant to your application including details of what the award was for.
2.5 ORCiD
Lead Applicants must include an ORCID iD in their application. Without it, your application will not be validated and you will not be able to submit. View more information on ORCID, including how to register.
2.6 Publication record
Please download this NIHR Research Professorships Publication Record document. Please do not list all publications in the ‘Manage My Details: Update CV’ section.
Peer reviewed publication summary
Please provide details of the most significant 20 original, peer-reviewed publications that would be of relevance to this application from the last 5 years. Please list in date order, with the most recent first.
Only 20 publications will be considered, please do not add more than 20 publications.
Please set out your publications in the following order:
- Publication – please state the publication title.
- Journal – please state the journal that the output was published in.
- Year – please state the year published.
- Current Journal Impact factor – given that these change, please list the most current Impact factor for the journal listed.
- Number of Citations – please list the number of times that the publication has been cited.
- Source of Citation Information – please state where you obtained the citation information for each publication, e.g. Scopus, Google Scholar etc.
- Number of authors – please list the total number of authors associated with the publication.
- Key Authorship Position – please state your authorship position for each publication, e.g. first, last or corresponding author.
- Interdisciplinary research – please choose either yes or no from the drop down list.
- URL – please provide the web link to the journal (where available).
Other significant publications or outputs
Please provide details of any other significant publications, outputs or products that would be of relevance to this application, which provide evidence of influence and leadership in your field.
Items in this section should include the following headings (where appropriate):
- Books and/or book chapters;
- Papers in conference proceedings;
- Research reports to government departments, charities, the voluntary sector, professional bodies, industry or commerce;
- Monographs;
- Intellectual Property (patents, patent applications or other forms of IP);
- Other applied research outputs (new materials, software packages, images and devices, research derived from development, analysis and interpretation of bio-informatic databases, work published in non-print media, Cochrane reviews and NICE Guidelines) or equivalent for Global Health such as WHO SAGE.
Please complete the overall summary tables at the start of this section. Please state whether you have used Web of Science, Google Scholar or Scopus as the source of the information. Please do not use any other system.
Once completed, please upload onto the online application system. If you do not upload the publications document your application will be deemed to be incomplete and will not be processed.
Section 3: Applicant Research Background
3.1 Professional background
Select the one option which best describes your professional group. This will determine the options that appear below for your professional background.
3.2 Describe your research career to date - 1000 word limit
Please use this question to describe your research experience and career to date and how this makes you suitable for this award. The following items should be included, however please note that this is not an exhaustive list:
- Track record of research and its translation;
- Track record of driving collaboration and partnership in research’
- Contributions towards research leadership;
- Developing translational research capability;
- Track record in training researchers;
- Previous involvement of patients/service users, carers and public in research.
Please do not duplicate any information that will be included within the Detailed Research Plan section. Please do not include any web links, as they will be disregarded by the Selection Committee.
The option to upload one document for this section is only for the inclusion of charts, diagrams, tables and images that relate to the text in this section. Any other information/documents uploaded here will be removed. The document must be in either Word or PDF format.
Please do not leave this section blank on the online system in order to upload a document with text and images combined, as this will be removed and your application will be deemed incomplete.
Impact of Professorship
Please describe the impact this Professorship will have on your career, noting that this should provide you with a step change in your career path.
3.3 Has this application been previously submitted to this or any other funding body? 500 word limit
This includes previous submissions for an NIHR research training award, even if the proposed research has changed.
Please detail the title of any previous submissions, the funding body and scheme, the outcome and the date this is due if a decision is pending.
If the application was unsuccessful please indicate why and detail how this application differs from previous submission(s) and how any feedback received has been used to inform this application.
3.4 Contextual Factors - 500 words
Detail any mitigating factors the Selection Committee should be aware of. This could include:
- Career breaks due to parental leave, or periods of illness.
- Reduced time spent undertaking research due to a disability or caring responsibilities. This could include any physical or mental difficulty that may have impacted your research career. These are situations that have a significant impact on your ability to undertake research.
- Reduced opportunities to career support e.g. mentorship, and limited opportunities to undertake prior research and training.
- Impact of the COVID-19 pandemic on your research career.
Please also use this section to detail any other factors that may have impacted your research career not listed in the examples provided. The impact on your career to date will be specific to your particular circumstances but could include such impacts as limited opportunities to obtain grant funding, or fewer publications. In general terms, contextual factors should be significant, and relevant.
NIHR acknowledges that you may be reluctant, or uncomfortable disclosing relevant information that is sensitive. However, you should bear in mind that we are unable to take into account factors that you do not disclose. Please be assured that information provided by you is sensitive and will be treated confidentially and in line with General Data and Protection Regulations (GDPR).
Section 4: Plain English Summary of Research - 600 word limit
A plain English summary is a clear explanation of your research.
Many reviewers use this summary to inform their review of your funding application.
The reviewers will include those who do not have specialist knowledge of your field of research.
If it is felt that your plain English summary is not clear and of a good quality then you may be required to amend it prior to final funding approval.
You may wish to involve patients/service users, practitioners, carers and members of the public to develop a plain English summary. Consider including the following:
- aim(s) of the research
- background to the research
- design and methods used
- patient and public involvement/community engagement and
- involvement
- dissemination
The plain English summary is not the same as a scientific abstract.
For further information on writing in plain English take a look at our guidance for Plain English summaries.
Section 5: Scientific Abstract - 500 word limit
The scientific abstract should be a clear and concise scientific summary of the Detailed Research Plan / Methods. You may wish to include the following:
- Research question
- Background
- Aims and objectives
- Methods
- Timelines for delivery
- Anticipated impact and dissemination
Applicants may find the guidance on the EQUATOR Network website useful.
Section 6: Detailed Research Plan - 5000 word limit
6.1 Detailed Research Plan - 5000 word limit
This is the main section considered by the selection committee. Please ensure the information is accurate, succinct and clearly laid out. You should consider the information below when answering the 10 questions within your research plan.
When completing this section please do not include web links, as they will be disregarded by the Selection Committee.
You can upload only one document for this section. This document can contain charts, diagrams, tables and images that relate to the text in this section. The document must be in either Word or PDF format.
Please do not leave this section blank on the online system in order to upload a document with text and images combined.
The research programme should have clear patient/service user benefit by the end of the award. All implementation plans need to be clearly defined and need a Gantt chart setting out what will be achieved and when should be included within the uploaded document for this section. If you are including a feasibility study, you should undertake and complete this within the duration of the 5 years of the award (ideally within 1 year) and then move the research forward.
Community Engagement and Involvement (CEI)
The NIHR expects the research it funds to involve patients, service users, carers and the public and other key stakeholders. You must set out how you will involve these stakeholders.
You must detail all relevant stakeholders to your research proposal. For each stakeholder group, you must detail how each group will benefit from your research, how they will be involved in the proposed research and if appropriate how they were involved in your application.
Your CEI plans will be assessed by the Selection Committee including patient and public members.
For NIHR Global Research Professorships, there is no standard model for CEI. You will need to explain why you have chosen your approach for your proposed research, who will be involved and why.
Research Inclusion for study participants
Every person eligible to take part in research should be offered the same opportunity of taking part in that research regardless of:
- Age
- Disability
- Gender reassignment
- Marriage and civil partnership
- Pregnancy and maternity
- Ethnicity
- Religion or belief
- Sex
- Sexual orientation
- Geographical location
- Socioeconomic status
- Access to health or social care
You should demonstrate how these factors have been considered and addressed in your proposal, including steps taken to ensure the research sample is representative of the population the study is targeted at.
You will need to complete an Equality Impact Assessment. where you can explain who you are planning to recruit to ensure inclusivity of study participants and any exclusions.
NIHR is committed to promoting Equality Diversity and Inclusion in research.
Format of the Detailed Research Plan
In this section you will detail your future research and translational plans over the 5 year time period. Please include a case for how an NIHR Global Research Professorship would enable you to make a significantly increased contribution towards translation into practice and developing translational research capability.
Any applications involving basic research or work involving animals or their tissue going forward will be deemed ineligible.
Please do not replicate any information that has already been included within this application form.
Service delivery
If you are in a clinical or service delivery role, the majority of the duration of the award will be spent on research. However you should continue with service delivery to ensure research ideas are pulled into clinical, public health or social care practice.
You will be funded to continue to spend the equivalent of up to one day per week delivering service directly.
If you are not in a clinical or service delivery role, an equivalent time collaborating with others to facilitate service delivery directly in the relevant LMIC or have relevant collaborations can be supported.
Question 1: What is the problem being addressed?
- You should provide a clear explanation of:
- What is the health or care problem that your research addresses?
- How would your research impact on patients/service users, carers as well as health and care services?
- How does your research fill a demonstrable evidence gap?
Question 2: Why is this research important in terms of improving the health and/or wellbeing of the public and/or to patients/service users, carers and health and care services?
- You should identify:
- The health and care need(s) your research meets or contributes to.
- Outline the anticipated value or contribution the study will provide.
Question 3: Review of existing evidence - How does the existing literature support this proposal?
You should explain why this research is needed now, both in terms of time and relevance.
We will only fund primary research where the proposed research is informed by a review of the existing evidence.
Question 4: What is the research question / aims and objectives?
You should summarise the research question/key aims and objectives.
Question 5: Project Plan
Here you should provide an expert description of the project plan of investigation plus any additional points required to support statements made in the previous sections.
You should include any key references required to justify the points made such as the use of particular outcome measures or methods of analysis.
If applicable you should include: study design, justification of sample size, selection and exclusion criteria, methods of data collection and analysis, and justification for your choice of methodology.
Summary of patients/service users/carers/public as research participants
Your study should successfully recruit and retain participants. If your proposed study involves patients/service users/carers/public as research participants, you should use the following bullet points to summarise the participant’s characteristics and what would be expected of the participants throughout the research project lifecycle. The potential burden on study participants can then be understood as well as whether or not the proposed strategies are practical, inclusive and feasible. Please also signpost to where further information on these points can be found in the detailed research plan and application.
Points to Cover:
Inclusion and exclusion criteria to help ensure that certain groups were not being excluded without justification
- Recruitment method and consent process to ensure it is practical and fair
- Type and content of participant information materials
- Overview of research methods to capture data from participants and their frequency e.g. questionnaires/tests/intervention/focus groups/ interviews
- Study participant support to consider how drop-out and issues of participation would be handled/helplines/ other access arrangements required
- Methods for sharing study progress and findings with study participants
- Payments, rewards and recognition for study participants.
- Setting/context: Please describe the health or social care service setting or context in which the study will take place (such as the organisation or service type).
Question 6: Dissemination, Outputs and Anticipated Impact
Here you should describe what the outputs of the research might be, how and who you will talk to and what impact there might be. NIHR understands that the impact of any research may take time to be realised and will likely involve other funders and institutions. In many cases it may be difficult to provide definitive answers or any guarantees. However, addressing the below questions will allow you to describe what you hope or expect the pathway to impact to be, what might prevent impact and who else might be involved.
What do you intend to produce from your research?
This could include but is not limited to: Conference presentation or other workshop events; Publications (academic or otherwise); Guidelines (clinical, service or otherwise); Other copyright (e.g. questionnaires, training aids, toolkits, manuals, software, etc.); New or improved design of medical devices or instrumentation; New or improved diagnostic; Trial data that could be used to support a CE mark, market authorisation or equivalent; Trial data that could be used to shape or influence a healthcare or social care market or government; Potential new drug or healthcare intervention; Other. Please provide brief details of each of the anticipated outputs.
How will you inform and engage patients/service users, carers, relevant health and care system and the wider population about your work?
Describe your plans for disseminating this research. If you have not yet made plans, please outline at what stage in your project you intend to start formulating these.
How will your outputs enter the health and care system or society as a whole?
Describe how any new or improved outputs generate through the proposed research will be recognised, captured, managed and use directly in the health and care service or wider society. This might be through commercial exploitation or other non-commercial routes or means. If the output(s) from your research are likely to be commercial, describe the proposed route to market and by whom, or how you plan on developing this.
What further funding or support will be required if this research is successful (e.g. from NIHR, other Government departments, charity or industry)?
This should be linked to the responses in questions 2 and 3 above.
What are the possible barriers for further research, development, adoption and implementation?
Will the proposed research use data, technology, materials or other inventions that are subject to any form of intellectual property protection (e.g. copyright, design rights, patents) or rights owned by another organisation(s)? If yes, provide brief details including how such third party IP will be accessed (e.g. collaboration agreement, drug supply agreement).
What are the key current and future barriers to uptake of any likely output or innovation directly in the health and care service, through commercial exploitation or other means, e.g. potential regulatory hurdles?
What do you think the impact of your research will be and for whom?
In describing the anticipated impact of the expected outputs on the health and care of patients, the public, and on health and care services, please consider; patient, service user or carer benefit; changes in the relevant health and care system service (including efficiency savings); commercial return (which could contribute to economic growth). Indicate the anticipated timescale for the benefits to reach patients, the public and services, providing a quantitative estimate of the scale of these potential benefits, if possible.
Question 7: Project Management
Please outline the processes that will be put in place to ensure the research described will be well managed. It should be clear the length of time you propose to spend in each partner country and a clear justification for this. This should complement your research timetable upload. View section 10 on uploads.
Question 8: Ethics
Please describe any ethical and/or regulatory issues your research project raises and how you intend to address these. Research requiring ethical approval must have the appropriate approvals in place before it can commence. If there are no plans to obtain ethical review, this must be clearly justified. (Note that work outlined in your application/protocol must adhere to the Research Governance Framework).
Further guidance on the approval process is available from the Health Research Authority (HRA). The MRC and the HRA have designed a tool to help you decide whether you need ethical approval.
In addition to seeking independent/institutional ethics committee approval for planned research from your HEI or Research Institute, NIHR Global Research Professors should seek ethical review from an appropriate ethics body in each of the LMICs in which there are study participants. Where an appropriate ethics body does not exist, this should be highlighted clearly in your application or raised separately to the NIHR. Applicants may wish to refer to the MRC guidance on research involving human participants in LMICs when preparing their description of any ethical and/or regulatory issues your research project raises and how you intend to address these.
Question 9: Success Criteria
Please set out the measurements of success you intend to use and also the key risks to delivering this research and what contingencies you will put in place to deal with them. You should identify appropriate actions that would reduce or eliminate each risk or its impact.
Question 10: Research Capacity Strengthening
You must include a research capacity strengthening (RCS) component in your application.
NIHR recognise that there is no single agreed definition of RCS but highlight the UK Collaborative on Development Research (UKCDR) definition of RCS as ‘Enhancing the ability and resources of individuals, institutions, and/or systems to undertake, communicate, and/or use high quality research efficiently, effectively, and sustainably’.
For NIHR Global Health Research Programmes, the objectives and priorities for RCS identified by/with local stakeholders should support or bring sustainable solutions to key national problems within LMICs. Teams should consider and cost activities that will build and help sustain both researcher capacity and support wider finance and research management capacity at institutional level.
Funds can be requested for a range of activities, for example:
- Full or partial formal training posts (including but not limited to BSc, MSc, MPhil, MRes, PhD, Post docs);
- Training in technical research skills, training and support to facilitate effective Community Engagement and Involvement, and personal development skills, such as, but not limited to, grant writing, writing for publication, communication and influencing skills, time management, team working etc.;
- Other wider institutional capacity strengthening activities such as finance management, research management, data management, legal compliance and assurance training.
- Institutional systems for coaching, mentoring and/or peer-mentorship.
NIHR will also fund student fees for LMIC national students who are based in ODA-eligible countries but registered at an institution in a High-Income Country (HIC). In cases where the application includes LMIC student fees at a HIC institution, it is expected that the relevant Lead Applicant will negotiate with the HIC institution for reduced fees for the LMIC candidate. Applications for funding should show evidence of fees being reduced.
Please note: HIC studentship fees or stipend costs are eligible only for ODA-eligible students. Student fees or stipends for HIC students registered at an HIC institution would not be eligible, regardless of the programme of study.
Individuals receiving funding from, or being supported by, an NIHR Global Health Research Programme (including NIHR Global Research Professorships) to develop their academic career become NIHR Academy Members and are eligible for the career development and training support provided by the NIHR Academy.
Applicants may find the following resources useful:
- Seven principles for strengthening research capacity in low- and middle-income countries: simple ideas in a complex world (2014)
- UKCDR Research Capacity Strengthening Tools, Resources and Guides
- UKCDR Research Capacity Strengthening: Lessons from UK funded initiatives in Low and Middle Income Countries
- The Concordat to Support the Career Development of Researchers
6.2 Provide a statement on the ODA eligibility of the proposal including which countries on the DAC list and the populations within these countries that will primarily benefit from the research. - 500 word limit
Please use this section to demonstrate Official Development Assistance (ODA) compliance criteria and outline:
- Which country or countries on the OECD DAC list of ODA-eligible countries will directly benefit?
- How the research plan is directly and primarily relevant to the development challenges of those countries?
- How the outcomes will promote the health and welfare of a country or countries on the DAC list?
Where all or part of the research is not undertaken in an ODA-eligible country during the course of the award (including where a country graduates from the DAC list during the lifetime of the award or there is a need for specialist expertise) the application must clearly state the reasons for this with due consideration to the benefit of the research to ODA-eligible countries.
Section 7: Community Engagement and Involvement
NIHR is committed to supporting Community Engagement and Involvement (CEI) that empowers communities and fosters co-production of research. Involving communities in LMICs who are affected by the health challenge you are working on will improve the reach, quality and impact of your research. Please read about this commitment on our community engagement and involvement webpage.
7.1 Please describe how patients/service users and the community have been involved in developing this proposal - 350 word limit
You should describe who has been involved and why this is appropriate, what role(s) they have played and what influence or change has happened as result of their involvement.
Patients and the general public within a given community, especially vulnerable groups who are at the greatest risk, will normally be the key group included in CEI activities. Community stakeholders such as community leaders, opinion leaders, non-governmental organisations and civil society, service commissioners and providers, policy and lawmakers are examples of other stakeholders who can be involved.
There is no standard model for CEI. You should demonstrate that your CEI approach is appropriate and effective in the local context and for their study design.
7.2 Please describe the ways in which patient/service users and the community will be actively engaged and involved in the proposed research, including any training and support provided - 350 words limit
CEI approach, management and support
- Explain why your approach to community engagement and involvement is appropriate for this proposal. In your description you will need to say who will be involved and why.
- Please use this opportunity to describe how you plan to manage and coordinate the CEI activities in your project.
- Describe how you will support and enable patients/service users, carers, the public and members of relevant communities to contribute to your research (e.g. access, payments, training).
- We would also encourage you to outline plans for the capturing, evaluating and reporting the impact of CEI activities.
A summary of CEI activities
Please provide a summary below of the proposed CEI activities embedded throughout the research project lifecycle. Please clearly signpost to other sections of the Detailed Research Plan where the CEI is described further in relation to the relevant project stage e.g. dissemination, intervention design, data collection, analysis.
Applicants may find the following information and resources helpful:
- NIHR Community Engagement and Involvement Resource Guide
- UNICEF Minimum Quality Standards and Indicators for Community Engagement
- NIHR and Institute of Development Studies (IDS) CEI learning resource
- Involvement payment guidance for researchers
Applicants may also find it helpful to refer to Mesh, a collaborative open-access web space that provides resources, encourages networking and shares good practice to bridge the gap between the research community and the general public in low-and middle-income countries.
There is also further guidance available in the NIHR Global Health Research core guidance.
7.3 If it is considered not appropriate and meaningful to actively involved patients/service users and the community in your proposal, please justify why - 350 word limit
Complete and justify why it is considered not appropriate and meaningful to actively involved patients/service users and the community in your proposal. Only complete this section if it is relevant.
Section 8: Leadership and Development and Research Support
8.1 Proposed leadership and development programme - 1000 word limit
You will be expected to participate in a leadership and development programme to support your personal and professional development as a research leader. Please detail your existing skills and outline a tailored development programme, which has been designed in conjunction with your nominating partnership organisations to address your future needs. Please provide a clear statement of how the HEI or Research Institute will actively support and develop your leadership and development programme. A strong leadership and development programme will focus mainly on the skills required for progression as a research leader. Successful NIHR Global Research Professors will also be invited to participate in the NIHR Leadership Programme. As this award may be used in part to build on existing national and international research leadership and collaborations, please include details of any sabbaticals that you may wish to undertake during the 5 years of the award, for consideration by the Selection Committee.
Please detail how you intend to develop research capacity in the next generation of researchers in the LMICs and UK (if applicable), including via your support post appointments and the wider environment. Please also state clearly where you expect to be on your career trajectory after the 5 years of the award and also what commitment the HEI or Research Institute intends to make towards your future career.
8.2 Collaborations - 600 word limit
Explain what collaborations you intend to establish to support your research and, if applicable, your leadership and development programme. This may involve short visiting placements (e.g. an International Research Visit), or secondments in new (to the applicant) research environments, e.g. clinical trials units or other NIHR Global Health research projects.
For the NIHR Global Research Professorships, collaboration with researchers in OECD DAC list countries is essential. Youwill be required to have existing strong collaborations or links with collaborators or partners in institutions in countries on the OECD DAC list and the award should plan to strengthen these as well as support training and capacity development/mentorship in these institutions.
It is expected that the principles of equitable partnership will be embedded in the NIHR Global Research Professorship applications across all stages of the research process from research design to dissemination and publication. Where applicants are building on established partnerships, involvement of individuals and organisations based in the relevant LMIC(s) in developing the research proposal and in undertaking the research should be clearly set out.
Further guidance on this is available in the NIHR Equitable Partnerships Guide.
8.3 Research Support - 250 word limit per justification of each proposed mentor
Please state the name(s) and institution(s) of your mentors. You will also be required to justify your choice of mentor and how you intend to work with them. A minimum of 1 and a maximum of 4 entries are required on the form.
Although we acknowledge that formal supervision may not be appropriate for the level of award, we believe that senior academic mentoring is vital to allow your development as a research leader. In this context, the mentoring role will encompass providing you with support throughout the Professorship in both your research endeavours and your overall career development.
At least one of the proposed senior academic mentors should be based in the host institution. They should have a clear understanding of the research process, the demands that the chosen area of research are likely to place on you, and your particular strengths and weaknesses. You may also wish to choose another mentor from another institution, including those in other countries.
Please state why these particular mentors have been chosen and what new perspective/ developments they will bring. Clearly describe how the proposed mentorship will support your overall development and provide an initial assessment of the time that will be allocated to the mentoring process.
Funding for research support is available for travel and subsistence only (for the nominee) and does not support any fees the individuals who provide research support may wish to charge the nominee.
8.4 Host Organisation Statement of Support - 1000 word limit
To be completed by the Lead Signatory of the host organisation.
It is the responsibility of the applicant to ensure that each point below is covered in the statement of support. Additionally, you may be required to clarify these points or update the details of the support being offered by the host, at interview.
The nominating host organisation will need to include within this statement when they expect the applicant to be appointed as a Professor (full Professor, not Associate or Assistant) and, if already appointed at Professorial level, why they consider the applicant is still qualified for this award and how the award will change their potential to become a leader in the field.
The lead organisation must state how the interface with service will be managed to empower the applicant to promote translation for applicants who are health professionals, or how the partnership intends to collaborate with service/practice in the case of non-health professional applicants. Please note that NIHR Global Research Professors will be funded to continue to spend the equivalent of up to one day per week delivering service directly. If you are not in a clinical or service delivery role, an equivalent time collaborating with others to facilitate service delivery directly in the relevant LMIC or have relevant collaborations can be supported.
Please state clearly how the partnership intends to work together to support the applicant and support post appointments, including how they will be supported in terms of infrastructure in the short, medium and long term.
Please note that this statement of support will need to be individually tailored to each applicant that the partnership wishes to be considered for a NIHR Global Research Professorship, rather than a generic institutional statement. These statements are critically important and will be used by the Selection Committee to determine whether the support from the host environment for the applicant and the proposed research is suitable enough to allow the application to progress beyond the shortlisting stage of the process.
In addition, the statement should provide evidence of the host organisation(s) commitment to creating and maintaining an inclusive and supportive research culture, including evidence of commitment to the principles of equality, diversity and inclusion and research integrity.
Statements may wish to refer to the principles and best practice outlined within relevant Charters and Concordats in these areas, such as the Researcher Development Concordat and Advance HE’s Equality Charters. For UK HEIs, it should be noted that being a signatory to Concordats or holding bronze/silver status from the Equality Charters isn’t a requirement of funding and evidence can be provided through other means. LMIC HEIs or Research Institutes may wish to refer to relevant charters or other relevant institutional, national, or regional initiatives.
The Statement of Support must also contain, but is not restricted to, information on the following two items:
- Support for the applicant at an organisational level - Please provide a clear statement outlining the strategic significance and value of the applicant and their research to the host institution. The HEI or Research Institute must also set out how it specifically intends to value and actively support the applicant at the organisational level both during and after completion of this award.
- Support for the applicant at a national/international level - Please provide a clear statement outlining the strategic significance and value of the applicant and their research at a national/international level. The HEI or Research Institute must also set out how it specifically intends to value and actively support the applicant at the national/international level both during and after completion of this award
In addition, the host institution will be required to answer further questions clearly documenting what additional support will be provided. This will be used as part of the evaluation process and should the Selection Committee deem the support for the applicant to be insufficient, then it is highly unlikely that an award will be made.
8.5 For the recycling of the applicant's salary, please state how this will be used to support the applicant - 1000 word limit
To be completed by the Lead Signatory of the host organisation.
For the recycling of the applicant's salary, please state how this will be used to directly support the applicant . This will be required for all applications with a UK HEI as host but we are aware circumstances may differ for applications with a LMCI HEI or Research Institute around ability to recycle salary. Please note that Global Research Professorships are designed to relieve applicants of onerous teaching, administrative and managerial duties so details of how this will be done can be explained here.
8.6 Please state what additional support will be given to the applicant over the short, medium and long term. - 1000 word limit
To be completed by the Lead Signatory of the host organisation.
Please state what additional support will be given to the applicant over the short, medium and long term.
8.7 Partner Organisation Statement of Support - 1000 word limit
To be completed by the signatory from the partner organisation.
In addition to the statement of support from the host organisation, the main Partner Organisation must provide a separate statement of support and to provide the selection committee with a clear confirmation of the partners’ commitment to the applicant.
Section 9 - Detailed Budget
9.1 Justification of Costs
- Please provide a breakdown of research costs associated with undertaking the research and provide justification for the resources requested. This should include the following costs: staff costs, travel and subsistence, dissemination costs, equipment (including lease versus purchase costs), consumables, CEI and any other direct costs.
- When justifying staff costs you should also provide the % amount of time input of each member of staff and link this to the specific area/work package of the proposed study where this input will be taking place.
- You should indicate here how this research will potentially benefit healthcare, social care, or public health in the relevant LMIC(s). For example, where appropriate, describe the likely cost savings or benefits in terms of numbers of patients treated, treatment times, service users or carers supported etc.
- You should describe the value for money of the conduct of the proposed research.
- NIHR Personal awards are not project or programme grants; therefore, extensions to the duration of awards to allow for completion of research and/or training and development are not permitted. This doesn't affect suspensions of awards to allow for periods of maternity, paternity, adoption or sickness leave.
9.2 Detailed Budget Breakdown
The finance section should provide a breakdown of costs associated with undertaking the research as described in the proposal.
General Information
- The information entered in this section should provide an analysis of the total funds requested to undertake the research proposed and should be based on current prices. These costs will be used to assess value for money.
-
The average cost of a Research Professorship is between £1.7m to £1.8m. Costs may be slightly higher or lower than this depending upon the research being undertaken and the location where the research is taking place; however any project over £2m will need to be re-profiled to bring it under this figure
-
It is in the best interest to undertake a thorough, realistic and accurate costing. You must provide a clear and full justification for all costs including NHS costs. You must also ensure that you include all costs including those required to secure good research management.
-
Costs must be provided at current prices. An adjustment for inflation will be made annually thereafter at rates set by the Department of Health and Social Care. Whilst allowances for incremental increases should be included on the form, nationally or locally agreed pay increases should be excluded.
-
Years should be calculated starting from the anticipated start date of the proposed research. For example, if your research is expected to start on 01 October 2024 then its second year starts 01 October 2025.
-
Further itemisation of costs and methods of calculation may be requested to support the application at a later date.
-
Payments will be made to the contracted organisation only and the contracted organisation will be responsible for passing on any money due to their partner organisation(s).
-
Appropriate sub-contracts must be put in place for any element of the research which is to be paid to another organisation.
-
All applications are expected to have appropriate HEI or Research Institute, commercial and other partner organisation input into the finance section of the application form.
-
There is no need to individually itemise costs where the total is below £1,000.
Further information can be found in Frequently asked questions regarding finances for NIHR Global Research Professorships.
9.3 Information for Different Types of Organisations
LMIC Higher Education Institutions (HEIs) and Research Institutes:
For applications that include contracting or collaborating organisations based in LMICs (as listed on the OECD DAC list), 100% of direct and indirect costs will be funded.
UK Higher Education Institutions (HEIs):
UK Higher Education Institutions (HEIs) should determine the Full Economic Cost (FEC) of their research using the Transparent Approach to Costing (TRAC) methodology. For UK HEIs, up to 80% of FEC will be paid, provided that TRAC methodology has been used.
Other Partner Organisations
If you are another partner organisation (e.g. charity or NGO), please fill in direct costs and other partner organisations indirect costs. Indirect costs should be charged in proportion to the amount of research staff effort requested on the funding application form. Up to 100% of costs will be paid.
9.4 Direct Costs
These are costs that are specific to the research, which will be charged as the amount actually spent and can be supported by an audit record. They should comprise:
Details of posts and salaries
This section presents an overview of salary costs for the applicant and other support/shared staff contributing to the research, including normal salary increments broken down individually.
The Applicant
Please state your proposed salary point and scale at the start of the Professorship. Please note immediate promotion to a higher grade as a result of securing an award will not be funded. Please do not include any Clinical Excellence or Discretion/Merit awards or discretionary points. NIHR agrees to fund consultant salaries at a full-time rate equivalent to 10 Programmed Activities per week.
Support staff
Typically, three support posts are available to accompany the Professorships. We appreciate that the team required for the Global Research Professor will vary depending on the local research community and may be a mixture of post-doctoral, doctoral and pre-doctoral posts. We have provided an example below of support posts for reference. It is also an option for successful applicants to review the requested support posts at the point of award. In any case, applicants are advised to speak to NIHR for guidance if they are unsure what would be appropriate.
- Post-Doctoral (5 years) – this could be for one person at Post-Doctoral level. Where this post is employed by an LMIC HEI or Research Institute, under ‘Type of Cost’, please enter this post as ‘Other’ from the drop down list Where this post is employed by a UK HEI, under ‘Type of Cost’, please enter this post as ‘HEI’ from the drop down list. This post attracts Full Economic Costing and has a training allowance of £3,000. Any conferences, courses etc. must be included within this £3,000 limit.
- Post-Doctoral (3 years) – this could be for one person at Post-Doctoral level. Under ‘Type of Cost’, please enter this post as ‘Other’, even if the post will be based in a UK HEI. This post does not attract Full Economic Costing and will therefore be paid at 100%. If you choose HEI, it will be returned at 80%, rather than the 100% required. There is no training allowance for this post.
- Doctoral Post (3 years) – this could be for one person at Doctoral level. Under ‘Type of Cost’, please enter this post as ‘Other’, even if the post will be based in a HEI. This post does not attract Full Economic Costing and will therefore be paid at 100%. If you choose HEI, it will be returned at 80%, rather than the 100% required. There is no training allowance for this post. PhD fees for this post should be included within the Training and Development section.
All of the above posts, including any associated funding, will terminate with the end of the Professorship, therefore early recruitment is recommended.
Please include all members of staff working on the research by clicking ‘add staff details’ or editing a current one. Where applicants are already receiving salaries funded by NIHR, these should be declared in the application. Only the posts described above should be entered as ‘Support Staff’. All proportions of time for other staff should be added as ‘Shared Staff’.
Salary costs
This section specifies the annual costs of the applicant and other staff contributing to the research. You should now allocate the individual staff member costs to each year of the research, allowing for increments. Use current rates of pay, and build in any known annual increments (again at current rates). You will not be able to claim for pay awards retrospectively, once your research is underway.
Please note the salary figures need to be calculated using the current annual costs, % WTE and number of months. If the research lasts for several years and an individual’s involvement varies over the course, it may be necessary to explain fully in the justification of costs section the % WTE and months per year for an individual staff member.
It is important to double check that the % WTE, total months and yearly costs information are consistent with the information presented in ‘Details of Posts and Salaries’ (‘Details of Posts and Salaries’ should show the full current staff costs independent of % WTE etc., whereas the yearly costs in ‘Salary Costs’ depend on % WTE etc.).
Please ensure that you check the ‘Type of Cost’ box which describes the employing organisation for a member of staff as this impacts on the level of funding provided. Staff employed by a UK Higher Education Institution (HEI) are funded at 80% of cost and staff employed by LMIC HEI, research institution, commercial or other partner organisation at up to 100% of cost.
Please note that this section also includes ‘Shared Staff Costs’ which is located under directly allocated costs in some other funders’ applications. These are costs of an institution’s research resources which can be charged to the research on the basis of estimated use, rather than actual costs. These may include: IT technicians, laboratory staff, and costs of pooled staff efforts. HEI indirect costs cannot be claimed on these shared costs. It is expected that no more than 20% of each individual’s time can be claimed as shared staff costs.
The NIHR reserves the right to question any costs deemed excessive, and will not fund:
- Contributions for individuals providing research support (previously referred to as mentors), supervisors and/or other collaborators involved in the research
- Administrative or secretarial support
- Whole or significant percentages of support posts over and above those permitted by the scheme
- Technical or research support staff whose costs are funded through institutional indirect costs (HEIs only)
9.5 Travel, Subsistence and Dissemination costs
This section includes journey costs, subsistence and dissemination costs, including conference fees and open access publication costs. Where applicable, you will need to include the travel and subsistence costs of your Project Advisory Group, Steering Committee and/or Data Monitoring & Ethics Committee. Travel and subsistence costs relating to dissemination should also be included here, as should costs relating to overseas travel. Where applicable, you will need to include the travel and subsistence costs relating to meetings with individuals providing research support. Please note that mentors’ (including supervisors and individuals named as providing research support) expenses will not be funded.
If a cost relates to travel, subsistence or fees for a conference please select ‘conference’. Costs relating to conference attendance will be funded at up to 100% for all employing/host organisation types. Conference costs don’t need to be individually itemised for each conference. The justification box should detail the conferences the costs will cover.
Journey Costs
Enter the total cost of transport for all journeys for destination/purpose. If travel is by car, apply your institution’s mileage rates (however this should not exceed His Majesty's Revenue & Customs (HMRC) approved mileage allowance payments, which is 45p per mile for the first 10,000 miles and 25p thereafter).
Travel by the most economic means possible is encouraged. NIHR programmes do not usually fund first class travel.
Subsistence
Subsistence covers accommodation (if necessary) and meals associated with the travel, excluding any alcoholic beverages.
Conference Fees
There is a limit on the amount that can be spent on conference related costs (including all related travel and subsistence as well as conference fees) depending on the level you are applying for.
Where national or international conference fees are included, a statement naming the conference or purpose of travel and the benefit to the Professorship must also be made; failure to adequately justify your attendance at a conference, will mean the programme will not fund this cost.
NIHR will fund a maximum of £15,000 for all conference-related costs for the NIHR Research Professor only.
Travel, subsistence and conference fees may not be claimed for the two 3 year Support Posts. For the 5 year Support Post, any travel, subsistence and conference costs must come from the £3,000 allowance.
Dissemination costs (in addition to conference costs)
- Open access costs: During the course of your project and throughout the review and publishing phase you may choose to submit an article based on your research to an Open Access publication. Depending on the publication you may be subject to an article processing charge (APC). APC rates vary but are usually within the range of £300 and £3000. Open Access publications usually list their APC rates on their websites.
- Where possible you should include an estimate for any APC in your funding application, since NIHR expects that APCs will be covered by the funding award.
- Other Other Dissemination Costs: Any large costs should be further detailed with a breakdown of constituent parts or a timescale profile of the costs. Meetings to share best practice, training events and events to disseminate research findings must be run at the lowest possible cost with minimal catering. ‘Conferences’ which are described as such are not eligible for funding.
Equipment
Essential items of equipment plus maintenance and related costs not included as part of estates should be input in this section. These can be lease or purchase costs. The purchase cost of pieces of equipment, valued up to £5,000 excluding VAT, will be considered.
Pieces of equipment costing more than £5,000 to purchase will usually need to be leased. Where applicants are leasing equipment with a purchase price of more than £5,000, a comparison of leasing verses purchasing costs must be provided in the ‘Justification of Costs’ section.
Items of equipment valued at £250 or more must be itemised separately; however grouping same type equipment is permitted. Costs of computers are normally restricted to a maximum of £1000 each excluding VAT and a statement of justification must be included, in the relevant ‘Justification of Costs’ section for any purchase above this limit.
Equipment must exclude VAT, but if your organisation is unable to reclaim/recover the VAT on a piece of equipment, you should check the box ‘VAT cannot be reclaimed’. You will need to seek expert advice from the organisation purchasing the equipment regarding its VAT status. If you check the ‘VAT cannot be reclaimed’ column, VAT at 20% will automatically be calculated into the overall cost of that item.
Consumables
This section includes non-reusable items specific to the research. Please itemise and describe the requirements fully (e.g. postage, stationery, photocopying). These items should be research specific, not just general office costs which should be covered by indirect costs.
Community Engagement and Involvement
Please note that in the detailed budget section of the application form there is no option to add “Community Engagement and Involvement costs”. Please add Community Engagement and Involvement costs as a “Patient and Public Involvement Cost”.
Please itemise and describe fully the costs associated with CEIt. These are likely to include individual travel, out of pocket expenses, payment for time and any relevant training and support costs. Costs related to study participants should not be itemised here.
If voluntary, charity or community groups are supporting the research via activities such as facilitating contact with potential participants, hosting research activities or providing advice, an adequate budget must be included to compensate for their time and resources.
Other Direct Costs
These are costs, not identified elsewhere, that are specifically attributed to the research. For example, costs associated with the use of research facilities, external consultancy costs, computer licensing, recruitment and advertising costs. Please note that for organisations claiming indirect/overhead costs, costs such as recruitment of staff, and general training (e.g. in common IT packages) are costs that should be covered by the indirect costs element of the award being sought and should not appear in this section.
If external consultancy costs are included in this section they must be fully justified in the ‘Justification of Costs’ section. Please specify the hourly rate and the number of hours and note that consultants must not be people who are already employed by the applicant’s institution. If they are, any costs should be entered as direct costs in the ‘Details of Posts and Salaries’ and ‘Annual Costs of Posts’ sections.
Note on Clinical Trial Unit (CTU) costs in Personal Training Awards
Costs claimed should be for the additional support from the CTU for the necessary expertise that the applicant cannot provide themselves. For example, part time support from a trial manager, database manager, and statistician are all costs that could potentially be included. The level of support and input from the CTU will likely vary depending on the level of fellowship and experience of the applicant. For example, doctoral applicants will be expected to be undertaking the majority of the day-to-day tasks involved in running a trial, with oversight from a more senior member of CTU staff (though specialist input in database programming may be needed). For more senior post-doctoral awards it may be more appropriate for other members of staff to be undertaking some of the day-to-day tasks. This also very much depends on the experience and expertise of the applicant and the applicant’s training needs and should be agreed with the CTU before submitting an application. These costs should all be agreed with the CTU and budgeted for. Staff costs should be detailed under the ‘other direct costs’ section. Staff costs should include basic salary and on-costs for each member of staff involved and it should be made clear within the justification section what role each member of staff has within the context of the personal award application and the time they will spend on the award. Please note that because NIHR Global Research Professorships are personal awards and not project or programme grants we can’t fund whole or significant portions of posts other than that of the applicant themselves and their support staff member (where applicable). We would not normally expect the time commitment of any individual costed into the application other than the applicant or member of support staff to exceed 0.3 WTE. In total we wouldn’t normally expect the total WTE of all staff costed into the application to support clinical trial activities to exceed 1 WTE (excluding the applicant and support staff member) for more junior awards (doctoral and early post-doctoral level awards) and 2 WTEs for more senior awards (this includes any shared staff also costed into the application). The level of additional staff input will obviously depend on the type and scope of the trial and the experience of the applicant. Full justification should be provided for all staff costs requested. Overheads(estates and indirect costs) can be included for CTU staff costed into the application. The justification section should split out the overheads from the salary costs and overheads shouldn’t exceed 40% of the total CTU staff cost.
Any costs must be realistic in order to deliver the trial but must also represent value for money. Applicants can also include non-staff costs for the CTU for example; randomisation service, and licence fees for clinical data management software.
Training and Development
All costs in this section will be funded at up to 100%. Please itemise and describe fully the costs associated with training and development. Please provide estimates if exact costs are not available at the time of application. Any travel and subsistence associated with training and development including overseas research visits should not be included here and should be included in the travel section of the form.
Leadership Training Programme, short course and workshops
These are costs relating to your training programme.
Support Post – Training & Development (5 year Post-Doctoral position only)
NIHR will make a maximum contribution of £3,000 in total (including any identified travel and subsistence) for the duration of this Professorship towards the cost of training and development for the member of staff in the 5 year support post.
While recognising that the NIHR Global Research Professorship primarily represents an investment in an individual, NIHR expects to see consideration of training for those researchers based in LMICs to support capacity building.
Whilst training and development costs for these awards are restricted to the NIHR Global Research Professor and the five year Support Staff post, the NIHR welcomes any additional contributions from host partnerships to the training and development costs for all Support Staff.
Overseas Research Visits
Please provide costs for any overseas research visits that the nominee wishes to undertake during the course of the award. The NIHR will consider overseas research visits on an individual basis and reserves the right to limit expenditure.
Support post – PhD fees
For UK based posts, these are capped at the current UKRI rates. For LMIC based posts, full PhD fees will be funded. In cases where the application includes LMIC student fees at a High Income Country (HIC) institution (often charged at higher rates for international students), it is expected that the Lead Applicant will negotiate with the HIC institution for reduced fees for the LMIC candidate to bring them in line with those paid by nationals. Application for funding should show evidence of fees being reduced. NIHR would require justification where only partial or no LMIC student fee reductions have been achieved.
9.6 Indirect Costs/Overheads
HEI Indirect Costs
Total HEI indirect costs must be fully justified. HEIs are permitted to claim estate and other indirect costs. These costs are calculated on the basis of TRAC methodology. Proposals from other types of institutions/organisations should leave this section blank.
HEI indirect costs are based on the number of full-time equivalent research staff working on the research and the indirect/estates charges set by an institution. Please note HEI indirect costs cannot be claimed on shared staff costs. Where staff from more than one HEI are working on the research there may be different indirect/estates charges for each one. Please list each institution on a separate line.
The applicant(s) should consult their HEI Finance Departments for the appropriate figures to include in the estate charges and other indirect cost sections
Commercial/Other Partner Organisation Indirect Costs
Commercial/Other Partner Organisations can claim indirect costs which are the costs of resources used by the research that are shared by other activities. Please seek advice from your finance department about the appropriate cost for this section.
Total Commercial/Other Partner Organisation indirect costs must be fully justified.
Indirect Costs
Indirect costs will be charged in proportion to the amount of research staff effort requested on the award. Commercial/Other Partner Organisations should calculate them, using their own cost rates. They comprise:
- General office and basic laboratory consumables
- Premises costs
- Library services/learning resources
- Typing/secretarial
- Finance, personnel, public relations and departmental services
- Usage costs of major research facilities
- Central and distributed computing
- Charge out rates for shared equipment
- Cost of capital employed
Summary of Costs
- NIHR programmes currently fund UK HEIs at a maximum of 80% of full economic cost.
- Please note that whilst these percentages will be used to calculate the maximum grant payable, the programme reserves the right to award a grant for less than this maximum where it is considered appropriate.
9.7 Frequently asked questions regarding finances for NIHR Global Research Professorships
Are budget headings flexible?
We will allow requests for relevant flexibility within the budget, particularly with research cost headings such as equipment, consumables, travel, CEI and other direct costs. Successful applicants can make requests during the contracting phase and also during the award should the circumstances require flexibility around the configuration of the budget. Please note that strong justification must be made for any requests to make changes to the budget.
Can funding be disbursed to partner institutions in OECD DAC-list countries?
Yes. We strongly encourage partnerships with LMIC researchers and institutions and would welcome this, at the discretion of the host HEI or Research Institute. The host will act as the contracting organisation, meaning that it will be the recipient of the funds, and will ultimately be responsible for the delivery of the research. All funding must be routed through the host and it will be the responsibility of the host to undertake appropriate due diligence and instigate appropriate and relevant financial controls for any funding to be disbursed to other organisations.
All research costs need to be justified in the application and should reflect the principles of equitable partnerships. Guidance on equitable research partnerships can be found on the NIHR Global Research webpage.
If the host is UK based, how should the funding be split between the UK institution and any LMIC organisation?
Payment will be made to the contracted UK HEI only and the UK HEI will be responsible for passing on any money due to their partner organisation(s). There are no set guidelines as to the percentage breakdown of funding between partnering organisation and any funding split should be discussed and agreed between the UK HEI and the partner organisation(s). However, your research must meet the ODA compliance criteria and all research costs should be justified in the application and reflect the principles of equitable partnerships. Guidance on equitable research partnerships can be found on the NIHR Global Research webpage.
If the host is UK based, will the award fund staff in LMICs?
Yes, staff employed to work in partner LMIC institution(s) may be included in the costings. Where they will be working in the three ‘Support Staff’ posts they should be entered in the Direct Costs as ‘Support Staff’.
The proportions of time for all other staff at LMIC institution(s) who are contributing to the research should be added as ‘Shared Staff’. ‘Shared Staff’ costs are costs of an institution’s research resources which can be charged to the research on the basis of estimated use, rather than actual costs. These may include: IT technicians, laboratory staff, and costs of pooled staff efforts. HEI indirect costs cannot be claimed on these shared costs. It is expected that no more than 30% of each individual’s time can be claimed as ‘Shared Staff’ costs.
Each team member, whether Support or Shared Staff, will need to be fully justified and reasonable consideration given to how much time they will be spending on the associated work.
Can overseas allowances be claimed?
Yes, if the NIHR Global Research Professor and any Support Staff are required to relocate for the purposes of this award, relocation expenses will be considered, provided that these are costed at standard rates according to the host institution’s policy. If the host institution does not have a policy for these allowances, then advice should be sought from the NIHR Academy.
Will travel costs be supported?
There is some flexibility for travel between the country of the host organisation and relevant LMIC(s) for the NIHR Global Research Professor and all Support Staff. For example, regular visits may be required to oversee the research being conducted in different countries, maintain involvement in the conduct and progress of the research and provide training and mentorship.
The NIHR Global Research Professors can travel internationally for research, collaboration and to ensure support and supervision. Support Staff can also travel internationally where there is a clear rationale and research need for them to do so. Any costs related to this must be fully justified in the application.
What is considered “value for money”?
The NIHR Global Research Professorship programme considers good value for money as the optimal use of resources to achieve the intended outcomes. ‘Optimal’ being considered as ‘the most desirable possible given expressed or implied restrictions or constraints’. Value for money goes beyond achieving the lowest initial price and includes consideration of Economy, Efficiency, Effectiveness, and Equity (as appropriate) and what these mean in the context of a research professorship application:
- Economy: Are we buying inputs of the appropriate quality at the right price? (Inputs are things such as staff, consultants, raw materials and capital that are used to produce outputs)
- Efficiency: How well do we convert inputs into outputs? (Outputs are results delivered by us or our agents to an external party. We exercise strong control over the quality and quantity of outputs)
- Effectiveness: How well are the outputs from an intervention achieving the desired outcome? (Note that in contrast to outputs, we do not exercise direct control over outcomes)
- Equity: the extent to which the outputs of our interventions are equitably distributed
NIHR may challenge proposed costs that it does not consider appropriate or does not offer value for money, for example Business Class travel.
Can large items of equipment over £5,000 be purchased under the award?
Essential equipment costs including maintenance and related costs not included as part of estates should be input in the ‘Detailed Budget Breakdown’ of applications under ‘Equipment’. For applications to the NIHR Global Research Professorship the equipment cost cap of £5,000 does not apply. Instead the proposed cost of all equipment to be purchased needs to be fully justified and all proposed costs should be good value for money. NIHR may challenge proposed costs that it does not consider appropriate or does not offer value for money.
Please note that equipment bought within the NHS cannot be funded by the NIHR. Please ensure no NHS equipment costs are included in your application.
Costs of computers are normally restricted to a maximum of £650 each excluding VAT and a statement of justification must be included in the relevant ‘Justification of Costs’ section for any purchase above this limit.
Equipment must exclude VAT, but if your organisation is unable to reclaim/recover the VAT on a piece of equipment, you should check the box ‘VAT cannot be reclaimed’. You will need to seek expert advice from the organisation purchasing the equipment regarding its VAT status. If you check the ‘VAT cannot be reclaimed’ column, VAT at 20% will automatically be calculated into the overall cost of that item.
Can full economic costs be claimed for both the UK HEI and OECD DAC-list partner institutions?
- UK based HEIs: UK based HEIs should determine the Full Economic Cost (FEC) of their research using the Transparent Approach to Costing (TRAC) methodology. For HEIs in the UK, up to 80% of FEC will be paid, provided that TRAC methodology has been used.
- HEIs or Research institutions based in LMICs: All costs including direct and indirect will be supported at 100%. Indirect costs must be fully justified as to why these costs are being requested and how they will contribute to the proposed research.
- Commercial/other partner organisations: For a commercial organisation/consultancy, please fill in direct costs and indirect costs. Indirect costs should be charged in proportion to the amount of research staff effort requested on the funding application form. Up to 100% of direct costs will be paid. The NIHR reserves the right to set limits on indirect costs charged. For other partner organisation (e.g. charity or NGO), please fill in direct costs and indirect costs. Indirect costs should be charged in proportion to the amount of research staff effort requested on the funding application form. Up to 100% of direct costs will be paid. The NIHR reserves the right to set limits on indirect costs charged.
Section 10 - Uploads
To support your research plan you are able to upload the following documents in the ‘uploads’ section of the form:
- References - 1 A4 page listing all references cited in the application.
- Figures/Tables - Up to 2 A4 pages of figures/tables may be included to supplement your research plan.
- Research Timetable - 1 A4 page detailing specific milestones and deliverables (Gantt Chart).
- CTU Letter of Support Where you are working with a CTU please include a supporting letter.
- Publications Summary - Please use the ‘Publications Record’ form downloaded from the NIHR website to include all relevant publication information.
A completed Schedule of Events Cost Attribution Tool (SoECAT) is required to be uploaded and submitted as part of the application submission if your research. Please insert a blank SoECAT form if one is not required for your research.
Section 11 - Participants and Signatories
A number of participants and signatories are required to be added to your application and, where applicable, to complete sections of it. Details of the required individuals are provided on the online application form along with details of how they should be added.
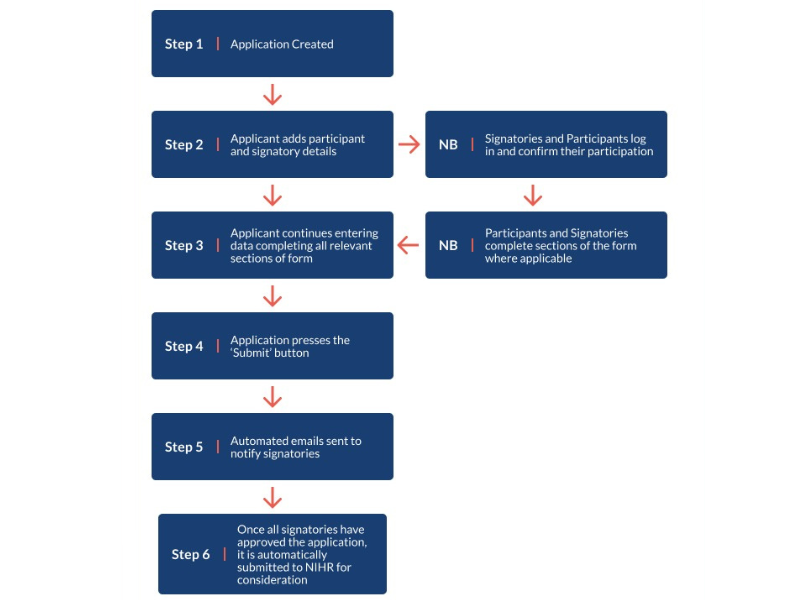
Image description: Application submission Process Flow Diagram Step 1: Application created, Step 2: Applicant adds participant and signatory details, Signatories and Participants log in and confirm their participation, Signatories and Participants complete sections of the form where applicable, Step 3 Applicant continues entering data completing all the relevant sections of the form, Step 4: applicant presses the submit button, Step 5: Automated emails sent to notify signatories, Step 6 Once all signatories have approved the application it is automatically submitted to the NIHR for consideration (rejection of the application by any individual at step 6 will return the application to Step 3).
Section 12 - Acknowledge, review and submit
12.1 Conflict Checks
Please declare any conflicts or potential conflicts of interest that you may have in undertaking this research, including any relevant, non-personal & commercial interest that could be perceived as a conflict of interest.
12.2 Agreement to terms and conditions
Please click the check box to confirm you agree to the Terms and Conditions of submission as detailed on the application form.
Section 13 - Checklist of information to include when submitting a NIHR research application
Applicants should use list below to check that they have included the necessary information prior to submitting their application:
- A good quality Plain English Summary
- A clear explanation of the problem being addressed
- A clear demonstration of the need and importance of the research
- A review of existing literature (primary research)
- A clear research question / aim(s) and objectives
- A clear project plan summarising the study design and methods
- Appropriate and relevant involvement of patients/service users, carers and the public
- A clear, appropriate and relevant plan for dissemination
- A single A4 page of references (document upload)
- All mandatory additional information has been uploaded to the online application system.
Section 14 - Additional Supporting Information
14.1 Background and Foreground IP
Please note that in relation to intellectual property, the NIHR would like the research team to clarify various key points in the application, namely:
- That it will have permission to use any background intellectual property for the purposes of the research and, if necessary due to complicated ownership arrangements, complete schedule C of the NIHR research contract with reference to clause 24.1.5 (a) of the NIHR ODA Global Health research contract.
- The proposed ownership arrangements of the Foreground IP, Arising Know How and Research Data. The parties should consider who is best placed to use, disseminate and/or commercially exploit the relevant intellectual property and/or database to maximise the opportunities to deliver patient benefit. There should be license arrangements with collaborators (usually contained within the Collaboration Agreement signed for a project) for research and teaching purposes and/or in the support of the development, promotion or provision of Health Care or for any other purpose that is not a Commercial Use.
14.2 Joint IP
With respect to joint IP, please note that the NIHR does not recommend joint ownership of Foreground IP, Arising Know How and/or Research Data arrangements preferring that the research team agrees the IP ownership arrangements at the application stage as above. This is because Joint IP (i.e. owning the IP jointly) can lead to delays about how the IP/data is used, disseminated and/or exploited and we want to avoid this potential barrier to delivering patient benefit. Normally, giving collaborators appropriate licensed rights to use Foreground IP, Arising Know How and Research Data is sufficient to avoid the need for joint IP. If specific circumstances for a proposed project mean that joint ownership of Foreground IP, Arising Know How and/or Research Data may be appropriate, please provide a justification in your application.
14.3 Commercial Use
In the event the relevant owner of the IP intends to commercially exploit any Foreground IP, Arising Know How and/or Research Data, then they must seek the NIHR’s prior written consent via IP.Unit@nihr.ac.uk and the relevant NIHR programme manager.
14.4 Plagiarism in NIHR funding applications
NIHR expects all content within applications for funding to be original material of the applicant's own work, with the exception of sections that other participants are required to complete. Whilst we anticipate and expect that applicants will get help and advice from various sources when putting together an application, including on occasion input from those previously awarded funding, care must be taken to ensure this does not lead to plagiarism of either published work or other previous applications. If an allegation of plagiarism is raised against an application this will be investigated in accordance with the NIHR policy on plagiarism, a copy of which is available on request from academy-awards@nihr.ac.uk.
14.5 NIHR Privacy Policy
Our purpose for collecting information is to communicate with you about your application and have the necessary information to evaluate you for a grant. The data we collect here is collected in the public interest. Information provided here may be subject to Freedom of Information requests.
The NIHR Academy is part of the Department for Health and Social Care (DHSC), National Institute for Health Research (NIHR). The contracting agent for the NIHR Academy is the Leeds Teaching Hospital Trust (LTHT). The DHSC is the Data Controller and LTHT is the Data Processor under the General Data Protection Regulation (GDPR) EC 2016/679. DHSC NIHR respects the privacy of individuals who share their data and processes it in a manner that meets the requirements of GDPR. The DHSC Data Protection Officer can be contacted by email at: data_protection@dh.gsi.gov.uk
The NIHR privacy policy includes further information including ways we may use your data, our contact details and details on your individual rights regarding how your data is used. Your data may be shared across the NIHR, including with other coordinating centres, to allow the application to be managed and for statistical analysis, and with external grant reviewers as part of the process for managing the allocation of a grant. Information collected from you will not be shared outside the EEA without your consent.
This notice is under constant review and will be updated and / or revised based on that review as appropriate.
International Standard Randomised Controlled Trial Number (ISRCTN)
All primary research studies need to be assigned an ISRCTN. Visit the ISRCTN website.
Please note that the remit of this database has been widened to include all primary research projects, even those that are not randomised controlled trials. There is no registration fee for NIHR funded trials.
14.6 NIHR Carbon Reduction Guidelines
Researchers applying for NIHR funding are asked to consider the carbon footprint of their research and take steps to reduce carbon emissions where appropriate. Advice on how to do this can be obtained from the NIHR Carbon Reduction Guidelines.
14.7 Transparency Agenda
In line with the government’s transparency agenda, any contract resulting from this tender may be published in its entirety to the general public.
14.8 Clinical Trials Unit (CTU) support
Applicants thinking of including a clinical trial, feasibility or pilot study as part of their application, or are undertaking research and/or training related to clinical trials are encouraged to consider working with a CTU where appropriate. Further guidance for and applicants is available in the NIHR Clinical Trials Guide for Trainees. This includes guidance on how to go about approaching a suitable CTU to support your application.
14.9 MRC Complex Intervention Guidance
Where appropriate applicants are encouraged to read the MRC complex interventions guidance.
14.10 NIHR Research Design Service
The NIHR provides support for prospective applicants to make high quality applications for research funding from the NIHR and from other national research funders. Assistance is primarily focused around refinement of research questions, research design and methodological support. Complementing the advice applicants receive from supervisors and/or mentors. The research support services can also assist prospective applicants to understand the scope of the NIHR’s various funding streams and to develop patient and public involvement (PPI) strategies. The Research Support Services may be able to support applicants with small grants to work up PPI plans with, for example, patient groups.
For applicants seeking support before 1 October 2023
Please contact the NIHR Research Design Service. The RDS has regional offices and links with local networks. View further information regarding the RDS.
For applicants seeking research support after 1st October 2023
From 1st October 2023, the Research Design Service will be replaced by the new Research Support Service. The RSS will be based on best match to research need or specialism rather than location. Please seek the advice of the Research Support Service.
14.11 Ethics / Regulatory Approvals
Research requiring ethical approval must have the appropriate approvals in place, usually in both the LMIC and UK (any other country involved in the research) before it can commence. Applicants should ensure all plans for research follow the UK Policy Framework For Health and Social Care Research, the Concordat to support Research Integrity (.PDF), and the UK HRA guidance Governance Arrangements for Research Ethics Committees, and that research performed in partner countries is conducted in accordance with regulations and to a standard no less stringent than those applicable in the UK. Applicants should provide their plans for ethical review of the proposed activities in the relevant countries (LMIC/non-LMIC). Applicants should anticipate that securing ethical reviews can be a long process in their work plans. NIHR will seek evidence of positive ethics approvals as part of NIHR's standard contract monitoring. If there are no plans to obtain ethical review, the reasons for this must be provided.
Where the research involves vulnerable individuals or groups (e.g. children under the age of 18 or individuals lacking capacity to consent), applicants should describe how they will manage their involvement in the Ethical Consideration section of their Detailed Research Plan
Applicants may find it useful to refer to the following online resources:
- MRC guidelines for the management of global health trials
- The Wellcome Trust’s guidelines on research involving people living in low and middle income countries
- The Health Research Authority (HRA)
- HRA tool to help you decide whether you need ethical approval
- The MRC guidance on ethics and approvals.
Section 15 - Example of host organisation support for a NIHR Global Research Professorship candidate
Should the Selection Committee consider the host support to be insufficient, then it is highly unlikely that an award will be made.
This example has been based on applications with a UK HEI as a host. We are aware host organisation support may look different for HEIs and Research Institutes in LMICs compared to what is listed in the example below and the selection committee will take this into account when making their assessments.
15.1 Question 1: Please state what additional support will be given to the applicant over the short, medium and long term.
In addition to recycling Professor X’s salary the partnership will provide:
Short term (1-3 years)
- Insert name of new piece of large scale equipment to be used by Professor X + additional computing system for data analysis.
- Refurbished additional office/analysis space adjacent to new equipment to accommodate staff.
- Full time clinical research nurse.
Additional research management support to co-ordinate meetings and correspondence with co-investigators and oversee regulatory and reporting requirements. - 1 PhD studentship.
- 1 Clinical PhD studentship.
- Clinical Governance support to help with study set-up/ethics and sponsorship.
- IT support to optimise patient management and database development.
- Research mentoring from high calibre academics within the partnership.
- Support for training and leadership through personalised development plans enshrining NIHR Professorship goals.
Medium term (3-5 years)
The partnership will continue with the same level of support outlined in the short-term in addition to:
- Creation of a clinical research facility in (insert name of specialty) that Professor X will direct.
- Appointment of a complimentary academic post at chair level and support posts, enhancing critical mass and interdisciplinary collaboration.
- Senior Leadership Training to compliment external programme outlined in the application.
Long term (5 years +)
Beyond the NIHR Professorship we will ensure continuity of support to Professor X’s research group befitting their standing through providing:
- 2 core funded support posts (HEFCE).
- Development towards theme lead/director.
- Institutional support towards any external appointment (e.g. charity/specialty specific chair).
- Continued investment in state-of-the-art clinical research facilities to ensure they have a world class environment in which to remain globally competitive.
15.2 Question 2: Where applicable, for the recycling of the applicant's salary, please state how this will be used to support the applicant
Professor X’s salary will be used to appoint a clinical senior lecturer who will work directly with them on the proposed projects. The new appointment will deputise as required for some of Professor X’s administrative duties, including on executive committees. The new senior lecturer will undertake lecturing duties in lieu of Professor X and will undertake supervisory roles for X-X clinical trainees per year, currently allocated to the applicant.
As the new senior lecturer post will be on a lower scale than Professor X, the remaining money will be used to increase the 0.5 FTE personal administrator support to full time.
These appointments will substantially reduce the burden of administration, teaching, supervision and regular meetings that will allow Professor X to focus on research and developing early career researchers.

