This document provides guidance for applicants completing an Expression of Interest form for the NIHR Invention for Innovation (i4i) Digital Health Technologies for Children and Young People’s Mental Health call.
You may also be interested in the supporting information.
Section 1: Introduction
1.1 Background
The i4i programme invites applications for research into digital health technology that addresses children and young people’s mental health. The i4i Programme supports research and development of digital health technologies, medical devices, active implantable devices and in vitro diagnostic devices to a point where they are de-risked for follow-on investment.
The i4i Digital Health Technologies for Children and Young People’s Mental Health (CYPMH) call encourages proposals addressing a range of children and young people’s mental health conditions particularly in regions that have been historically under-served by research activity or where there is high unmet mental health burden. The scope of the call is limited to diagnosable mental health conditions within the UKCRC Health Research Classification System - HRCS mental health category, including depression, schizophrenia, psychosis and personality disorders, addiction, suicide, anxiety, eating disorders, learning disabilities, bipolar disorder, autistic spectrum disorders and studies of normal psychology, cognitive function and behaviour.
Mental health problems are the single largest cause of disability in the UK and represent an ever-increasing burden across all ages of the population, and health and social care systems.[1] The impact mental health problems can have on individuals and the wider societal and economic consequences is vast and includes increased rates of co-morbid physical illnesses, shortened life expectancy, social exclusion, socioeconomic disadvantage, and the need for provision of health and welfare support.[2] Despite the high prevalence, many populations do not receive the support they need and there are significant inequalities to its access. Mental illness is increasing in the youngest members of society. Among children of primary school age (5 to 10-year olds), 14.4% had a probable mental disorder in 2020, an increase from 9.4% in 2017.[3] Seventy-five percent of mental illness begins before the age of eighteen.[4] The COVID-19 pandemic has also negatively impacted children and young people’s mental health. Significant future increase in poor mental health is predicted for adolescents and young children and action is needed to prevent a lasting, life changing impact on those affected.[5]
Mental health research is an ongoing priority for the Department of Health and Social Care (DHSC) and for the NIHR. Currently, a large proportion of mental health research activity is focused in a small number of geographical locations, with significantly less activity in areas where mental health conditions are most prevalent (see Appendix 1). The NIHR encourages research to be conducted in populations with high mental health burden which have been historically underserved by research activity or where there is an unmet need and, therefore, has an aim to address this disparity in mental health research.
1.2 Scope
To support this aim, the i4i Programme is inviting proposals on the research and development of digital health technologies aimed at improving children and young people’s mental health across England, with an emphasis on communities or regions with a high burden of common mental health conditions relative to ongoing research activity. The call encourages proposals addressing a range of mental health conditions, including but not limited to, work on the priorities identified by the Mental Health Research Goals 2020-2030 and the Right People, Right Question report.
Proposals should be designed for, and engage with under-served communities within mental health research, and aspire to reduce the demonstrated mismatch between mental health research need and the provision of evidence-based capacity for response. Proposals should provide a clear rationale for the selected region or population their research will be undertaken in, including how it is in particular need of mental health research relative to the rest of England, and the potential impact of the proposed activities for patients, care users, carers, the NHS, and social care.
To maximise the value of your data, we encourage you to use DATAMIND and make your data discoverable through the UK.
When preparing their proposals, applicants should seek to highlight the following requirements:
- How does the proposal address populations with high mental health and care need?
- How does the proposal build on existing evidence or practices within mental health?
- How does the proposal address stated regional or national priorities in mental health, and validate how they are of particular research need?
- Does the proposal clearly demonstrate how patients, service users and/or carers are involved and engaged in the research, and how the proposed research is relevant to them?
- Does the proposal include an appropriate range of multidisciplinary and methodological approaches?
- Does the proposal include clear plans for implementation, knowledge mobilisation and dissemination?
Section 2: Call Details
2.1 Essential Requirements
- Projects can be led by small-to-medium-sized enterprises (SMEs), NHS providers or higher education institutions (HEIs).
- Lead applicants must be based in England.
- Projects must be £50,000-£150,000 in value.
- Projects must be 6-12 months in duration.
- Projects need to have proof-of-concept evidence
- Projects need to address a clearly defined unmet clinical need around children and young people’s mental health, in accordance with the Scope of this call (Section 1.2).
- Project start dates must be by 01 January 2022.
- Upon acceptance of i4i funding, applicants agree to be bound by the terms of the NIHR standard research contract and are expected to sign the contract within 4 weeks of the award notice or the award may be withdrawn.
2.2 Eligibility
i4i Digital Health Technologies for CYPMH will fund:
- Research and development of Digital Health Technologies that fall under Tier C of the NICE Evidence Framework for Digital Health Technologies, and that are focused on mental health improvement and intended for ultimate NHS use. Evidence that the NHSX Digital Technology Assessment Criteria (DTAC) has been considered should be demonstrated in your proposal.
- Research and development of medical devices, as defined by the EU Medical Device Directive MDD (please see Regulating medical devices in the UK for details), across all mental health conditions, with a focus on treatment, and management of mental health.
- Generation of data to support an application to i4i Product Development Awards or any other funding stream.
- The activities eligible for funding are:
- Product design tasks, including but not limited to software development, user interface design and user requirement assessments.
- Intellectual property strategy, including freedom to operate analysis, Development of a commercialisation strategy and market analysis, Business case development
- Small safety and efficacy studies including clinical validation/utility studies
- Health economic model development or analyses
- CE/UKCA marking, where applicable, and other regulatory requirements, including any associated preparation for a future clinical investigation application
- Activities associated with the adoption of new technology and any training associated with the implementation of new technology
- Please note that the proposed projects should include a Research and Development component. Projects solely focusing on activities outside of these will not be funded, e.g. if the proposed work only involves business case development.
i4i Digital Health Technologies for CYPMH will not fund:
- Basic research
- Minor or incremental changes to technologies or interventions in current clinical use
- Projects involving small molecule drugs, stem cells or cosmetic products
- Projects that involve work on animals or animal tissue
- Evaluation or clinical trials of fully developed products or interventions, which have already been adopted within another NHS organisation or have a history of NHS use
- Studies on the impact of interventions on service delivery and management
- Methodologies clinically assessing or validating an existing or newly developed technique or technology
- Professional training
- Products to be used only in hospital information systems, administration, infrastructure and other related software, as per the NICE Evidence Framework classification for digital health technologies:
- All Tier A digital health technologies
- All Tier B digital health technologies
2.3 Applicant Eligibility
For all i4i awards the lead organisation must be based in England. The lead organisation may be any of the types of eligible organisation listed below. The following types of organisations are eligible for funding:
- SMEs (have a staff headcount no greater than 250 and annual turnover no greater than €50 million, including start-up or spin-out companies);
- NHS service providers (including Trusts, community care providers, and tertiary care centres);
- Universities, research institutes and not-for-profit organisations.
There is no requirement to have formed a collaboration prior to application; however, applicants seeking post-award i4i PDA funding will be expected to identify a collaborator/s as a deliverable during the project.
Specialist services or expertise may be brought into the team through consultancy or sub-contract arrangements, however, appropriate justification must be provided. Sub-contractors may be based outside of England if the required expertise or service cannot be reasonably contracted from within England.
We cover 100% costs for SME and NHS trusts and 80% FEC for academic partners. There are no set rules on the split between collaborators. However, as the contracted organisation, the lead applicant will receive funding payments and would be required to distribute to co-applicants/contractors where applicable.
Section 3: Assessment Criteria
Expression of interest applications will be assessed against the following criteria:
- Clinical need, health economic case and impact on the NHS and patients
- Level of innovation
- Quality of the project plan, including the technological content, and risk mitigation strategy
- Clear entry and exit points
- Strength of the project team and management arrangements
- Intellectual Property (IP) & commercialisation strategy
- Value for money
- Consideration of Patient and public involvement
Expression of Interest application forms include the following sections:
- Unmet patient need
- Proposed solution
- Clearly describe entry and exit points
- Benefits to patients and the healthcare system
- Project and Spending plan
- Applicant and team details
- Patient and public involvement (PPI)
Each of these sections must be addressed according to the guidance below. The maximum word count for each of these sections is 300 words.
Unmet Patient Need
Please provide details on the scale of the clinical problem to be addressed particularly in regions that have been historically under-served by research activity or where there is high unmet mental health burden, and on the limitations in current clinical practice. Refer to Section 1 for details.
Proposed Solution
Please provide a clear explanation of the technology, device or intervention and the benefits it provides over competing technologies. A consideration of the proposed barriers to clinical adoption must also be clearly articulated, as well as a description of the level of innovation of the proposed technology and Intellectual Property (IP) position. Technology entry and exit points need to be clearly stated.
Benefits to Patients and the Healthcare System
Please provide a clear case of how the proposed device, technology or intervention will change clinical practice and provide benefit to patients (such as reduced mortality or morbidity, improved quality of life, reduced misdiagnosis, improved patient outcomes and experiences). Potential cost savings for the NHS should also be provided.
Project and Spending Plan
Please provide details of the work to be conducted and how funds will be allocated. This section must adequately address the main objectives of the project. Project objectives must be realistic in terms of time and resources requested.
Applicant and Team Details
Arrangements for managing the project must be adequate and roles of team members must be clearly described. Project teams are expected to have included expertise in all areas relevant to develop the proposed device, technology or intervention to the expected project end point. If the applicant is an SME full details of the company are to be provided including company number, name, address and contact details. A summary of the company’s activities should be provided, including, if applicable, other products in development, any synergies of the proposed project with an already existing portfolio, or any other relevant information.
Patient and public involvement (PPI)
The NIHR expects active involvement of patients and the public in the projects it supports. It is anticipated that most i4i projects will have a significant PPI component, which must be clearly and fully described. Applicants should identify the relevant patient/user group(s) for their application and engage with those groups at an early stage.
Section 4: Application Process
i4i Digital Health Technologies for CYPMH will operate a two stage application process:
- Stage 1 – Expression of Interest
- Stage 2 – 15 page (maximum) Full applications and a 3 minute video pitch
The first stage requires applicants to submit an Expression of Interest form. Applicants shortlisted for Stage 2 will be required to submit a full application with a maximum of 15 pages and a 3 minute video pitch which will be reviewed by our i4i Digital Health Technologies for CYPMH Selection Committee. The Committee comprises commercial, clinical and academic experts.
Please note that this guidance document is for Expression of Interest applications only. Shortlisted applicants must refer to the i4i Digital Health Technologies for CYPMH Stage 2 guidance document which will be made available on the NIHR i4i webpage prior to Stage 2 launch.
4.1 Registration and Creating an Application
Applications to both stages of the process must be submitted online using the NIHR Research Management System (RMS). For Stage 1 Expressions of
Interest, the lead applicant must register on the RMS, providing contact details and a CV, in order to be able to create and complete the application.
To update your ORCID ID number, log in to the research management system, go to 'Manage my details' and select 'Update CV'. In this section you will be able to login to the ORCID ID site and create an account if you do not already have one. Your unique number will automatically pull through to your application form.
4.2 Validating and Submitting your Application
When the application form is complete it must be validated prior to submission. This will highlight any omissions in the form and allow these omissions to be corrected. After successful validation the lead applicant may submit the application. Once you have submitted your application, an i4i specific reference number will be generated. The application will be automatically considered for funding once the funding call closes.
4.3 Shortlisting
Assessment of EoI applications is carried out by the i4i Secretariat. Applications are scored against the EoI assessment criteria and shortlisted for Stage 2 at a meeting attended by the i4i Secretariat, the i4i Director and Chairman of the funding Committee. Successful applicants will be invited to Stage 2 of the process. Guidance for creating and submitting your full application for Stage 2 of the application process will be made available on our website at this time.
4.4 Confidentiality
Applications are treated as confidential and all steps are taken to ensure confidentiality is maintained. In line with the Department of Health and Social Care policy, i4i will publish summary minutes of the Selection Committee meetings. Please refer to our Confidentiality Guidance for further details.
4.5 Post Award Process
Funding Offer
Once your application has been recommended for funding, we will provide feedback agreed with the Committee. Successful applicants are expected to start their project by 01 January 2022, subject to satisfactory completion of due diligence and a fully signed contract. The contract must be concluded in December 2021 or the funding offer may be withdrawn.
Due Diligence
Due diligence is carried out by the i4i Secretariat as part of the funding process to highlight any potential areas of risk or weakness within a project. This allows for recommendations to address risks or weaknesses in the project and to identify the level of project monitoring that will be required. Projects recommended for funding will have to satisfy all conditions imposed by the Committee and the i4i Secretariat before the funding agreement can be put in place.
In addition to any changes to the work plan that may be requested by the Committee, further information may be requested on project finances, project management, intellectual property and commercialisation. Funded applicants may be required to engage with an independent advisor for the revision of the project plan or any other project elements.
A face to face meeting between funded applicants and an i4i Secretariat member is strongly encouraged to address key questions and allow quick conclusion of the funding contract.
Finances
The project finances will be scrutinised to ensure that all requested costs meet the NIHR finance guidelines, any costs that cannot be fully justified may have to be adjusted. The Department of Health and Social Care reserves the right to negotiate the price it is prepared to pay for the work, based on the cost of the application and its operating constraints.
For collaborative partnerships where a partner is providing in kind contributions, the exact nature of the commitment of each partner must be clearly detailed. SMEs and/or early-stage companies may be required to provide accounts and cash flow forecasts in order to demonstrate their capability to support a project throughout its lifetime.
Contracting
Once due diligence has been completed, the NIHR standard contract will be put in place between the lead organisation and the Department of Health and Social Care; this process will be managed by the NIHR. Applicants should refer to the terms and conditions under which the award will be made prior to applying for funding. These terms are set out in the NIHR standard contract and are non-negotiable. Upon acceptance of i4i CYPMH funding, applicants agree to be bound by the terms of the NIHR standard contract.
As part of the contracting process, a reporting and a payment schedule will be negotiated with the lead organisation, based on the proposed deliverables and milestones. The contract will be managed by the NIHR; all i4i projects will be actively monitored.
Section 5: Post Award Monitoring
5.1 Progress and Financial Reports
i4i will oversee the management and progress of funded projects based on the deliverables agreed in the contract. An i4i Programme Manager will be assigned to your project. We will use at least quarterly progress reports, email communication, phone calls and site visits to evaluate progress and the achievement of deliverables.
As payments will be made monthly in arrears, you will also be required to provide monthly expenditure reports and an annual statement of expenditure. Lead applicants are required to issue invoices at the end of each payment period; any deviation from the scheduled payment in the contract must be thoroughly explained. Any funding not spent at the end of the financial year may be recovered by the Department of Health and Social Care or set off against any future payments. In such situations, a new payment schedule will be issued.
5.2 Return on investment
The NIHR funds a wide spectrum of health research and is keen to support the exploitation of products or treatments developed under its funded research to ensure that the benefits are not lost to UK patients and there is a return on its investment. The return on investment will depend on the nature of the funded project and the level of funding provided and will be agreed as part of the NIHR commercialisation agreement. Potential forms of return on investment include:
- Patient benefit, such as reduced morbidity or mortality, and improvements in quality of life
- Cost savings, resulting from innovative practice methods developed within the public health and social care systems funded by the NIHR
- Commercial return in the form of a share of revenues generated through IP licensing or consultancy, taking shares in new businesses created, or seeking product or service discounts, thereby generating cost savings
- Public good, such as a demonstration of the impact of NIHR funding on the health and prosperity of the nation. When a project team wants to make commercial use of any IP generated during an i4i project, whether during the life of the project or at any time after the project has ended and is ready for commercialisation, written consent must be obtained by the Department of Health and Social Care and an income and/or equity-based revenue share will be agreed. We may consider requests for early agreement of commercialisation terms. At this time, the terms as set out in an NIHR commercialisation agreement will form the basis for negotiation.
Section 6: Key Dates
| Step | Date |
|---|---|
| Stage 1 Webinar | 13 July 2021 |
| Stage 1 (Expression of Interest) Launch | 17 August 2021 |
| Stage 1 Submission Deadline | 14 September 2021 |
| Stage 2 Launch | 06 October 2021 |
| Stage 2 Submission Deadline | 02 November 2021 |
| Submission Outcome | November 2021 |
Section 7: Contact Details and Further Information
We wish to ensure that potential applicants fully understand what is needed in their applications before they submit them. We encourage discussion of proposals prior to the deadline, although we cannot advise on the specific content of an application.
Enquiries may be made by contacting the i4i Secretariat by emailing i4i@nihr.ac.uk.
Appendix 1
Analysis of Regional Mental Health Burden and Research Activity
There is a mismatch between regional research activities (measured by patient recruitment per 100,000 patients) in mental health and the prevalence of mental health conditions in England in the past 10 years (2010-19). The recruitment per 100,000 prevalence map shows where the highest proportion of people with mental health conditions are being recruited to research studies (Figure 1).
The areas of highest prevalence per 1,000 map shows where mental health conditions are most prevalent, with the shade becoming paler as prevalence drops (Figure 2).
Research activity
Figure 1: Where are the highest proportions of people with common mental health conditions being recruited into mental health studies?
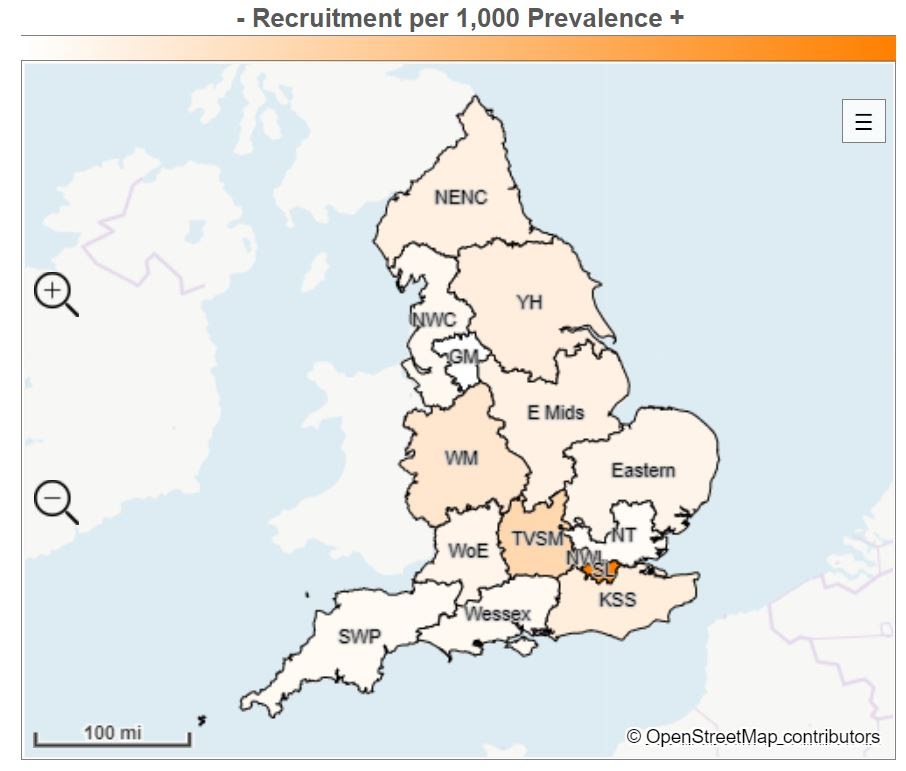
| Local Network | Recruitment | Prevalence | Recruitment per 1,000 Prevalence |
|---|---|---|---|
| South London | 22, 101 | 387, 025 | 57.1 |
| Thames Valley and South Midlands | 4, 332 | 222, 443 | 19.5 |
| West Midlands | 7, 484 | 568, 463 | 13.2 |
| North West London | 2, 869 | 250, 377 | 11.5 |
| Kent, Surrey and Sussex | 4, 286 | 426, 269 | 10.1 |
| Yorkshire and Humber | 6, 145 | 630, 356 | 9.7 |
| North East and North Cumbria | 3, 427 | 383, 540 | 8.9 |
| East Midlands | 3, 442 | 420, 127 | 8.2 |
| Eastern | 3, 108 | 418, 186 | 7.4 |
| West of England | 1, 888 | 265, 886 | 7.1 |
| North West Coast | 2, 929 | 521, 317 | 5.6 |
| Wessex | 1, 559 | 289, 753 | 5.4 |
| South West Peninsula | 1, 251 | 250, 971 | 5.0 |
| North Thames | 2, 803 | 648, 839 | 4.3 |
| Greater Manchester | 1, 133 | 430, 692 | 2.6 |
| England Total | 68, 757 | 6, 114, 244 | 11.2 |
Prevalence
Figure 2: Where are common mental health conditions most prevalent?
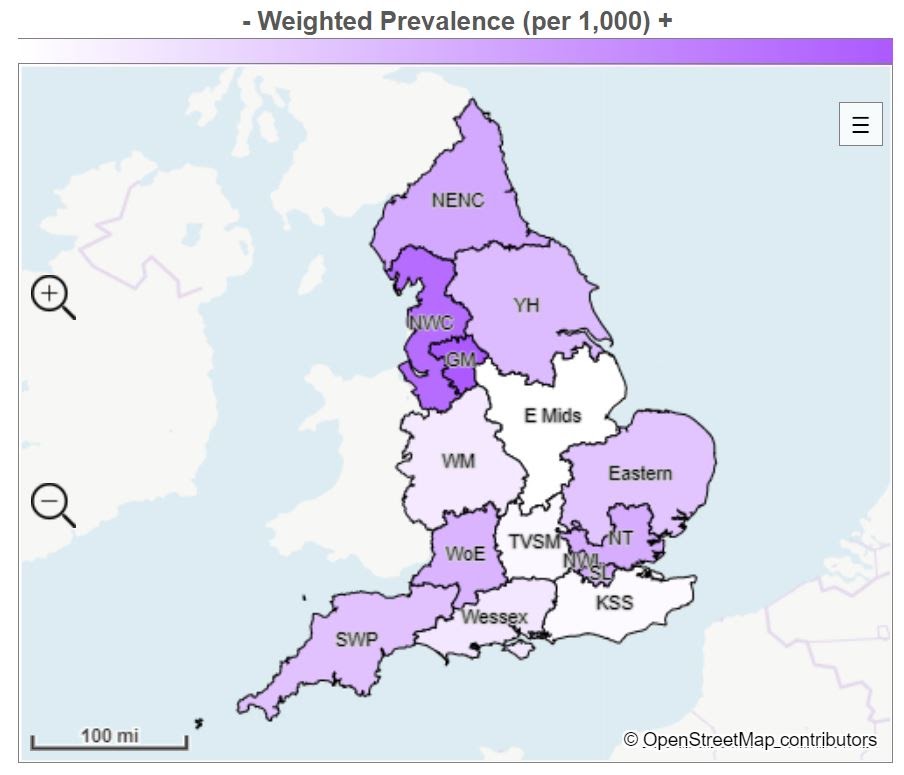
| Local Network | Prevalence | Adult Population | Weighted Prevalence (per 1, 000) |
|---|---|---|---|
| Greater Manchester | 430, 692 | 2, 146, 470 | 200.7 |
| North West Coast | 521, 317 | 2, 707, 490 | 192.5 |
| North East and North Cumbria | 383, 540 | 2, 296, 045 | 167.0 |
| North West London | 250, 377 | 1, 505, 994 | 166.3 |
| South London | 387, 025 | 2, 328, 617 | 166.2 |
| North Thames | 648, 839 | 3, 952, 295 | 164.2 |
| West of England | 265, 886 | 1, 644, 509 | 161.7 |
| Yorkshire and Humber | 630, 356 | 3, 971, 913 | 158.7 |
| South West Peninsula | 250, 971 | 1, 611, 110 | 155.8 |
| Eastern | 418, 186 | 2, 704, 008 | 154.7 |
| Wessex | 289, 753 | 2, 065, 847 | 140.3 |
| West Midlands | 568, 463 | 4, 088, 391 | 139.0 |
| Thames Valley and South Midlands | 222, 443 | 1, 673, 712 | 132.9 |
| Kent, Surrey and Sussex | 426, 269 | 3, 218, 657 | 132.4 |
| East Midlands | 420, 127 | 3, 236, 427 | 129.8 |
| England Total | 6, 114, 244 | 39, 151, 485 | 156.2 |
References
- Care Quality Commission. 2020 Community mental health survey: Statistical release. November 2020.
- Public Health England. Mental health: population factors. Mental health and wellbeing: JSNA toolkit. October 2019.
- NHS Digital. Mental Health of Children and Young People in England, 2020: Wave 1 follow up to the 2017 survey. October 2020.
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, & Poulton R. (2003) Prior Juvenile Diagnoses in Adults With Mental Disorder: Developmental Follow-Back of a Prospective-Longitudinal Cohort. Archives of General Psychiatry 60(7), 709–717.
- All-Party Parliamentary Group on a Fit and Healthy Childhood. The COVID generation: A mental health pandemic in the making. April 2021.

