This document provides information on the call specification for an application for Stage 1 for the NIHR Invention for Innovation Addiction Mission: Innovation for Treatment and Recovery (AMI) Awards.
The NIHR Invention for Innovation (i4i) Programme, in collaboration with the Office for Life Sciences (OLS), invites applications to the Addiction Mission: Innovation for Treatment and Recovery (AMI) Awards. Proposals must create valuable, innovative tools to help treat people with opioid or cocaine addictions and aid in their recovery.
The AMI Awards are a two-stage call inviting applications for projects up to 36 months in duration, with no upper funding limit.
The call will be open to all organisations which are UK legal entities, regardless of size or sector, that have an innovative solution to improve patient outcomes in addiction healthcare and recovery. For instance, this call is open to commercial entities, NHS and Third Sector Service Providers, charitable organisations, local government bodies, as well as universities and research institutions.
Introduction
The NIHR Invention for Innovation (i4i) Programme, in collaboration with the Office for Life Sciences (OLS), invites applications for an addiction healthcare-specific funding stream aimed at the research, development, and testing in real world settings, of innovative digital, MedTech, and pharmaceutical interventions that support treatment and recovery for people with opioid or cocaine addictions.
Innovations must focus on opioid or cocaine addiction. Innovations that are also applicable to other substances would be in scope only if they:
- primarily target opioid or cocaine addiction, and the focus of the proposed research project is to help treat people with opioid or cocaine addictions and aid in their recovery.
- support treatment or recovery for people with a pattern of polydrug use centred on opioid or cocaine addiction.
The Government published a new 10-year Drug Strategy, From Harm to Hope [1], in December 2021, responding to Professor Dame Carol Black’s Independent Review of Drugs [2]. This strategy sets out the Government’s high-level ambition to combat the harm caused by illegal drug use, create an environment that treats addiction as a chronic healthcare condition, and deliver a world-class treatment and recovery system.
The Addiction Healthcare Mission (the Addiction Mission), being led by OLS as part of the UK Government’s Life Sciences Vision (LSV), was announced in this strategy and is backed with £30.5m of government funding. As with seven other healthcare missions announced in the LSV, including Mental Health, Dementia, Cancer and Obesity, the Addiction Mission is aiming to bring together the best industrial and academic science, along with the wider sector, to significantly accelerate the development of new treatments and technologies.
The Mission aims to enhance the UK-wide research environment and incentivise the development of innovative and effective treatments, technologies, and approaches to support recovery, and reduce the harm and deaths which substance addictions can cause. This call forms a part of the strategy to deliver on this ambition following on from the previously launched Reducing Drugs Death Innovation Challenge which invited applications focused on preventing deaths from drug overdose events.
The call is open to all organisations which are a UK legal entity, regardless of size or sector. For instance, this call is open to commercial entities, NHS and Third Sector Service Providers, charitable organisations and local government bodies, as well as universities and research institutions.
Organisations can be based anywhere in the UK but the proposed research must show potential, and be appropriate, for roll out and use in all UK nations.
Collaborations, where relevant, with patient organisations, drug treatment services, recovery services, housing services, members of the public and employers, are strongly encouraged. Consultation with individuals who have current or lived experience is expected.
Support with research design and forging partnerships is available to all prospective applicants through the NIHR research support services, detailed below. All prospective applicants are encouraged to engage with these services at the earliest opportunity.
Background
Substance misuse is a major public health issue in England and the wider UK. As identified in Professor Dame Carol Black’s Independent Review of Drugs, there are an estimated 300,000 people in England using opioids and or crack cocaine and a number of challenges for treatment and recovery [2]. Addiction to illegal drugs costs the UK over £19.3 billion per year and causes huge societal damage and loss of life. Alongside the wider costs to society, the strain of managing a substance misuse issue can seriously damage a person’s work life and relationships and can have serious psychological and physical effects [3]. Treatment and recovery from substance misuse presents its own healthcare challenges and requires further support and understanding to better help substance use patients.
The Addiction Mission has identified three key challenges associated with the addiction treatment and research landscape in the UK. These include:
- an under-resourced and poorly connected translational patient research environment, with minimal involvement of industry to support research and development of innovations for addiction treatment and recovery;
- an insufficient range of novel treatment and recovery options with proven effectiveness, which highlights a need for developing and validating innovative approaches;
- and a limited evidence base for the causes of, and solutions to, drug addiction, particularly for those with co-occurring mental health diagnoses.
To address these challenges and realise the ambition of making the UK a leading location for innovative research into approaches to treat, and enable recovery from, substance misuse and reduce the harms and death it can cause, the Addiction Mission is delivering on two key areas:
- Transforming the UK’s drug addiction research ecosystem:
- better linking multidisciplinary researchers and treatment delivery partners with industry and innovators,
- enhancing research capacity and the ability to deliver novel patient research, and accelerating the development, testing and use of innovations targeting addiction.
- Funding innovation competitions to support UK industry and researchers, to engage in this market, to attract new innovators to the UK, and to catalyse the development and deployment of new and effective interventions that help to treat drug addiction, aid in recovery, or prevent drug misuse related harm and deaths.
On 31 January 2023, the Addiction Mission, in partnership with the Scottish Health Industry Partnership (SHIP) launched its first innovation competition. The £5m Reducing Drug Deaths Innovation Challenge which aims to develop innovative MedTech and Digital Health solutions that focus on detection of, response to, and intervention in, potentially fatal drug overdose episodes.
Following on from this, the launch of the Addiction Mission: Innovation for Treatment and Recovery Awards represents an opportunity for innovators to secure support for research, development, and testing in real world settings, of innovative digital, MedTech, and pharmaceutical interventions to help treat people with opioid or cocaine addictions and aid in their recovery.
Scope and Aims
The focus of the NIHR Invention for Innovation (i4i) Addiction Mission: Innovation for Treatment and Recovery (AMI) Awards is to create valuable, innovative tools to help treat people with opioid or cocaine addictions and aid in their recovery.
This call aims to:
- Increase the number of companies and innovators developing and testing products in the UK system and supporting the sector to grow and thrive through UK based research and development.
- Improve outcomes for people in treatment and recovery for opioid or cocaine substance misuse through accelerating development and availability of innovative digital health, MedTech and pharmaceutical interventions.
Types of intervention in scope for funding
Applications covering Digital, MedTech, and pharmaceutical interventions are all within scope.
The below list includes illustrative examples of interventions from which applications would be welcomed, but this is not an exhaustive list:
- Digital technology supporting interactive programmes to promote behaviour change and or maintenance for use by service users. For example:
- Treatment adjuncts: such as those which support delivery of supplementary treatment courses and content, monitoring of treatment and patient feedback, contingency management interventions, and/or approaches to encourage retention in treatment and the recovery journey.
- Structured, digitally delivered treatment: packages of drug focused interventions meeting care plan needs and goals of patients. This type of proposal would need to connect to trained healthcare professionals and include active interaction to meet clinical guidelines. These proposals could combine with multi-morbidity treatment pathways, for instance to align treatment for mental health conditions with addiction treatment.
- Relapse prevention/sustained recovery interventions: tracking, monitoring and feedback on ongoing medication and treatment plans, links to, or provision of mutual aid and support groups, tracking and flagging of relapse early warning signs, coaching sessions, or content to prevent relapse.
- Comprehensive assessment systems: to enable thorough remote assessment and determination of patient needs, optimal treatment pathways, and harm reduction strategies. These systems could provide opportunities to connect dual assessment of those with mental health conditions and substance misuse issues.
- Digital technologies within the above categories supported by Artificial Intelligence/Machine Learning (AI/ML), virtual reality and augmented reality, gamification, data analytics, or smartphone apps etc. would be welcomed.
- MedTech devices and solutions providing innovative or improved treatment delivery mechanisms for existing medicines. For example:
- Automated or timed dosing approaches (please note that innovations focusing on overdose events are not in scope).
- Novel administration approaches or routes leading to improved outcomes over existing approach in efficacy of medicine or patient adherence to treatment.
- Research and development of in vitro diagnostic devices as defined by the relevant EU Directives.
- Innovative combinations of interventions or products: Development, testing or validation of novel combinations in addiction healthcare. For instance, combining medications with psychological interventions delivered digitally.
- Pharmaceuticals for the treatment of drug addiction or to support in recovery including:
- Novel pharmaceuticals developed for substance misuse treatment or recovery at clinical trials (please see the entry point guidance for clinical trials).
- Licensed medicines or substances repurposed for substance misuse treatment or recovery.
Types of proposals in scope for funding through this call
- Product development required to enable a technology for clinical use; work packages may comprise aspects of prototyping and manufacturing, engineering and performance testing.
- Feasibility studies, and/or studies to provide data relating to safety and effectiveness of an innovation, including first-in-human and pivotal clinical studies.
- Activities associated with the design and delivery of evaluations of innovative technologies in real world settings.
- Clinical utility studies: usability, tolerability and user acceptance of interventions.
- Research and development of techniques and/or technologies from a different industry sector which could have a potential impact if applied in substance misuse treatment and recovery.
- Studies exploring the repurposing of existing medicines and/or interventions which could help treat substance misuse or support recovery.
- Studies exploring innovative combinations of interventions.
Eligible Entry Points
Proposals are welcome for innovations at the majority of development stages. Please see the schematic below which illustrates the eligible entry points for this call.
Although, strictly speaking, a demonstrated proof of concept is not required, the most competitive proposals are expected to show data and evidence to support the case for further development.
Basic research projects are out of scope.
For AMI we will assess proposal entry points using Technology Readiness Levels. Technology Readiness Levels (TRLs) are a way to describe the maturity of a particular technology. There are 10 TRLs; 1 being the earliest level of readiness, and 10 being a technology which is freely available and fully developed for market.
Digital and MedTech/Non-pharmaceutical innovations must be beyond concept stage, and ideally have a prototype ready to test outside the laboratory. Prototype development, however, is acceptable as part of the initial stages of the proposal but this must be beyond the conceptual stages. Eligibility is outlined as TRL-3 and above. If you are yet to develop your prototype and are unsure if you meet TRL-3, please contact the AMI Support Team for advice.
Pharmaceutical innovations evaluated by clinical trials are eligible from Phase One (first-in-human), with the exception of Phase Three trials. Phase Three trials are not eligible due to their size and expense, however eligible adjuncts to existing Phase Three trials are welcomed. Please contact the AMI Support Team to discuss whether your Phase Three adjunct project is eligible.
Entry Point Eligibility Schematic
Entry points in red are out of scope, those in blue are within scope. If your project falls within an orange entry point, please do contact the AMI support team to discuss eligibility.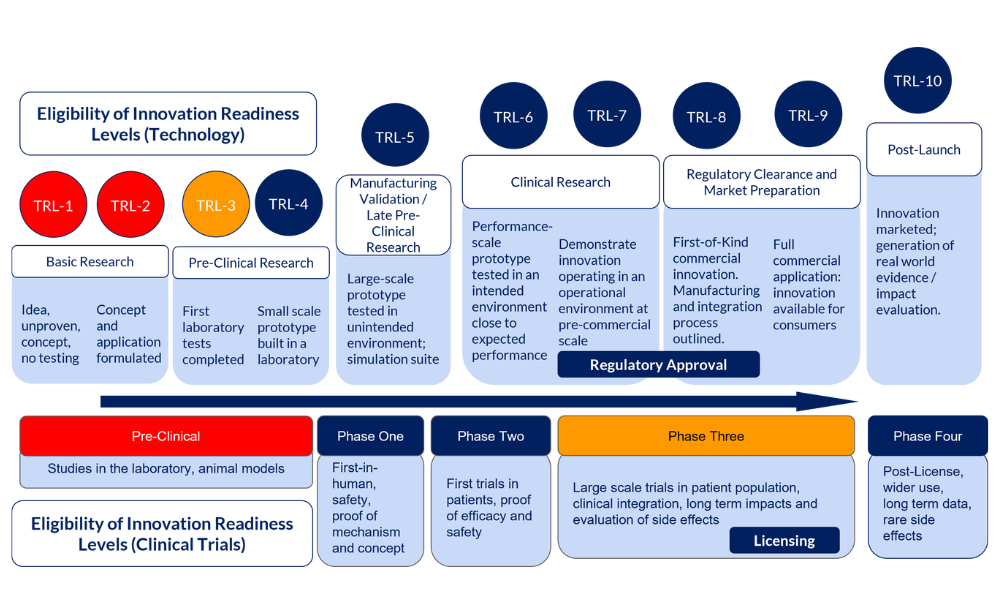
Entry Point Eligibility Schematic Description:
If your innovation is technology: Technology Readiness Levels (TRL)
- Not eligible (red)
- Basic Research
- TRL-1: Idea, unproven, concept, no testing
- TRL-2: Concept and application formulated
- Basic Research
- Eligible (blue)
- Available data/evidence to support the case for further development
- Pre-Clinical Research
- TRL-3: First laboratory tests completed
- TRL-4: Small scale prototype built in a laboratory
- Manufacturing Validation / Late Pre-Clinical Research
- TRL-5: Large-scale prototype tested in unintended environment; simulation suite
- Clinical Research
- TRL-6: Performance-scale prototype tested in an intended environment close to expected performance
- TRL-7: Demonstrate innovation operating in an operational environment at pre-commercial scale, gaining regulatory approval
- Regulatory Clearance and Market Preparation
- TRL-8: First-of-Kind commercial innovation. Manufacturing and integration process outlined, gaining regulatory approval
- TRL-9 :Full commercial application: innovation available for consumers
- Post-Launch
- TRL-10: Innovation marketed; generation of real world evidence / impact evaluation.
If your innovation requires a Clinical Trial: Clinical Trials
- Not eligible (red)
- Pre-Clinical: Studies in the laboratory, animal models
- Phase Three: Large scale trials in patient population, clinical integration, long term impacts and evaluation of side effects, licensing
- Eligible (blue)
- Phase One: First-in-human, safety, proof of mechanism and concept
- Phase Two: First trials in patients, proof of efficacy and safety
- Phase Three Adjuncts: Eligible adjuncts to an existing, current Phase Three Clinical Trial
- Phase Four: Post-License, wider use, long term data, rare side effects
Expected End Points
By the end of the funded period proposals should, as a minimum:
- Have created a prototype intervention (if not present at the outset of the project),
- Conducted service user or other human testing,
- Have developed a continued development pathway for approval and UK roll-out.
Funded proposals for interventions that are already in use, or in later stages of development at the outset of the project, will be expected to secure high-quality efficacy and use-case data to support wider UK uptake and use.
Areas out of scope
This call will not fund:
Ineligible activities:
- Proposals which do not primarily focus on outcomes for cocaine or opioid misuse treatment or recovery.
- Projects with no clear pathway to patient benefit.
- Work packages including animal or animal tissue studies. If animal or animal tissue studies are required as part of the project, we expect applicants to seek parallel funding to cover such studies, details of which should be provided in the application form.
- Proposals at Technology Readiness Level (TRL) 1-2 (concept and basic research stages) - please see the entry point eligibility schematic above.
- Proposals which focus only on in vitro diagnostics.
- Proposals for digital interventions that only provide health information, or only involve screening of patients without interactive, innovative involvement with treatment or recovery - such as treatment pathway determination and optimisation, data collection, storage or sharing.
- Wellness and/or Wellbeing applications unless specifically designed for opioid and/or cocaine addiction treatment and recovery.
- Phase Three clinical trials are out of scope, however eligible adjuncts to existing Phase Three trials are welcome.
Ineligible interventions:
- Interventions designed to prevent early drug use behaviour rather than treat, or aid in recovery from, existing addictions. These proposals are welcomed by the Innovation Fund to Reduce Demand for Illicit Substances Phase 2.
- Interventions that aid in detection of, and response to, or intervention in overdose events. These proposals were able to apply for funding through the SBRI Reducing Drugs Deaths Innovation Challenge.
- Development of new psychological treatment approaches, except where existing approaches are being adapted to be delivered via digital applications or technologies.
Call Details
Applicant Eligibility
Applications are welcome from all organisations which are a UK legal entity, regardless of size or sector, that have an innovative solution to improve patient outcomes in addiction healthcare and recovery.
- Applications are welcomed from both research institutions such as universities, and non-research institutions with a research arm such as commercial entities, NHS Service Providers, charitable organisations, and local government bodies etc.
- The lead organisation and collaborating organisations may be any of the types of eligible organisations listed below.
- Commercial organisations including large enterprises, SMEs, start-ups and newly spun-out companies;
- NHS service providers (including Trusts, community care providers and tertiary care centres, and Health and Social Care Trusts within Northern Ireland);
- Third Sector service providers;
- Primary care providers (including GP practices, primary care networks (PCNs) and GP Federations);
Please note that primary care providers should obtain formal sponsorship in order to apply for NIHR funding as the lead organisation, as per the Research Governance Framework - Higher education institutions (including universities and research institutes);
- Not-for-profit organisations (including charities and Community Interest Companies);
- Local Authorities
- Applicant organisations can be based anywhere in the UK but the proposed research must:
- take place in the UK and
- show potential, and be appropriate, for roll out across the UK.
- To support the growth of innovators in this field, applications from early career researchers and innovators are particularly welcomed. They can apply as either the lead or joint-lead applicant together with a senior colleague fulfilling the other role. We are keen to encourage fresh ideas from new innovators and appropriate applications are welcomed from those with limited research experience when supported by an experienced, strong and multi-disciplinary team.
- Collaborations, where relevant, with patient organisations, drug treatment services, recovery services, housing services, members of the public and employers, are strongly encouraged. Consultation with individuals who have current or lived experience is expected.
- Multidisciplinary project teams, involving relevant collaborations between technology developers, data scientists and clinical staff, are particularly welcome. We encourage clinical applicants to not only comprise clinicians involved in addiction healthcare, but also those involved in a target setting or in the care of at-risk patient populations. The lead organisation should be best placed among the project parties, to lead the research, and either own or have full access to the background IP.
- Specialist services or expertise may be brought into the team through consultancy or sub-contract arrangements; however, appropriate justification must be provided.
- Collaborators and sub-contractors may be based outside of the UK if the required expertise or service cannot be reasonably contracted from within the UK.
- Involvement of international companies as subcontractor and/or collaborator, if working with a lead UK partner, are welcomed.
Applicants must review the NIHR standard research contract before application submission, and agree in principle with its core terms and conditions as they are non-negotiable.
Budget and duration
- All funding proposals are expected to lead to projects of up to 36 months in duration.
- The call encourages proposals putting forward innovative solutions. To support this, there is no upper funding limit. We would expect the majority of applications requesting between £500,000 and £1.5m funding, however applicants are discouraged from compromising their ideas to meet this guideline, and applications will be considered on the overall merit and value for money they provide.
- NIHR funding covers 100% costs for commercial entities, not-for-profit organisations, local government bodies, and primary care providers: 100% of the direct research costs for NHS service providers, and 80% FEC for higher education institutions.
Support for Applicants and Awardees
Support with research design and forging partnerships is available to all prospective applicants through the NIHR research support services, detailed below. All prospective applicants are encouraged to engage with these services at the earliest opportunity.
- NIHR's Research Support Service - (previously supported by the Research Design Service) support on all aspects of developing and writing a funding application*.
- NIHR's Business Development Team - help with finding and making great partnerships to strengthen your work.
If you are considering applying and you are new to research or are not from a research institution, please do contact the appropriate NIHR research support services for free advice and support in finding potential research partners. This support is also available for experienced researchers, free of charge.
* Please note that the RSS is only available to applicants based in England. Applicants with a project partner based in England are able to access the service, but if you are based in the UK but outside of England, and are not partnered with an organisation based in England you may wish to explore the below services. The NIHR Business Development Team is available to all UK based applicants and you may wish to contact them in seeking a partner based in England.
- Applicants from Scotland may access The Drugs Research Network Scotland
- Applicants form Northern Ireland may access The Northern Ireland Clinical Research Network
- Applicants from Wales may access Health and Care Research Wales
Key dates and Contacts
| Information | Dates |
|---|---|
| Launch for Stage 1 applications: | 31 May 2023, 13:00 |
| Deadline for Stage 1 applications: | 12 July 2023, 13:00 |
| Notification of outcome of Stage 1: | 23 August 2023 |
| Launch for Stage 2 applications: | 23 August 2023 |
| Deadline for Stage 2 applications: | 11 October 2023 |
| Notification of outcome of Stage 2: | January/February 2024 |
| Projects start: | 26 February - 11 March 2024 |
The i4i AMI Awards programme team held a webinar for applicants on 31 May 2023. If you would like to view the webinar please contact AMI@nihr.ac.uk.
For any enquiries, please contact the AMI support team.
References
- From Harm to Hope: A 10-year drugs plan to cut crime and save lives 2021, accessed March 2023
- Independent Review of Drugs 2020, by Professor Dame Carol Black, accessed March 2023
- Addiction: what is it? NHS UK 2021, accessed March 2023

