The closing date for electronic submission of applications is 1pm GMT on 31 July 2019
Applications will be considered for up to a maximum value of £100,000 and up to nine months in duration.
It is mandatory for applications to the NIHR Global HPSR Development Awards to have two Joint Lead Applicants (one LMIC- and one UK-based). The NIHR contract requires the administrative ‘Lead Applicant’ to be from a UK based institution (UK administrative lead).
Joint Leadership must be demonstrated with plans in place for equal sharing of responsibilities. The applications will be assessed against scoring criteria which include the equity and strength of Lead Applicants and the proposed plans for development of the partnership.
How to apply
The NIHR uses a application system (REALMS) to receive applications. If you do not already have an account, you will be required to register on the system in order to access the application.
Once registered go to your homepage and click ‘Apply for Funding’ and choose GHPSR Development Awards from the list, click continue, then click apply.
Completing the online application form
Once you have clicked ‘Apply’ you will be taken to the main application page, you should check that you are applying to the right funding opportunity and call.
Please note: If you do not yet have a ORCiD you will be asked to create one here.
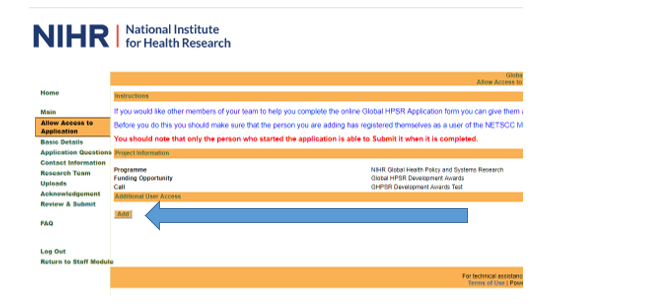
Allow access to application
UK Administrative Contact Details:
Do you wish us to contact the UK Joint Lead Applicant regarding this application? If no, provide other UK administrative contact details (name, post held, department, organisation, contact details and access rights).
Complete your name, contact details and other requested information.
You must ensure the ‘Permission Level’ is set to full edit access if you wish them to be able to make edits to the application on your behalf.
Basic Details
Project Title (300 characters):
The project title should state clearly and concisely the proposed research plan. Any abbreviations should be set out in full.
Brief Description (1000 characters):
Please give a brief high-level description of your planned study.
Please note: this section will be used on the NIHR website so please provide details you are happy to be shared there.
Proposed Start Date:
Note this should be from 1st of the month regardless of whether this is a working day or not. The Development Awards are expected to start on or before 1 March 2020 to align with future NIHR Global Health Policy and Systems Research (HPSR) funding opportunities; however, be realistic about your start date taking account of the necessary contracting and staff recruitment prior to starting your project. Changes to start dates will only be considered on a case by case basis.
End Date:
Please enter the end date of your proposed study.
Grant Request amount:
Please enter the figure calculated in your budget spreadsheet.
Application questions
Name of Proposed Host Institutions (150 characters):
Please give details of both the LMIC and UK Institution that will form your partnership.
Please note that we expect the UK Joint Lead Applicant’s host organisation (i.e. substantive employer) to act as the contractor/administrative lead if the project is funded.
Please also bear in mind that:
- Thought must be given to the most appropriate institution to act as the contractor as part of the application process, as changes are unlikely to be agreed once a funding decision has been made.
- Although the contract will be held by the UK institution for administrative purposes, there must be two Joint Lead Applicants (one LMIC and one UK) and clear evidence of equitable partnership between the LMIC partner, on the OECD DAC list, and the contractor.
- The contractor is expected to respond to quarterly reporting and annual financial reconciliation exercises, provide the final financial reconciliation statement for the project and to provide ad hoc requests for financial information during the lifetime of the project.
- In the same way, the contracting institution is expected to respond to any queries relating to, for example, Intellectual Property, commercialisation and benefit realisation.
Summary (in plain English) (3000 characters):
A plain English summary is a clear explanation of your plans/research.
Many reviewers use this summary to inform their review of your funding application. They include clinicians and researchers who do not have specialist knowledge of your field as well as members of the public. If your application for funding is successful, the summary will be used on NIHR and other websites.
A good quality plain English summary providing an easy to read overview of your whole study will help:
- those carrying out the review (reviewers and assessment committee members) to have a better understanding of your research proposal.
- inform others about your research such as members of the public, health professionals, policy makers and the media.
- the research funders to publicise the research that they fund.
If the plain English summary is not clear and of a good quality, you may be required to amend it prior to final funding approval.
It is helpful to involve patients / carers / communities or members of the public in developing a plain English summary.
Further guidance on writing in plain English is available online.
For further support and advice on writing a plain English summary, please contact your local Research Design Service (where applicable).
Summary of activities to be undertaken (10000 characters):
Please provide a summary of the methodologies and approach to undertaking your research/project. You must use the following headings to structure your response:
- Equitable Partnerships: Please give details of your approach to developing and supporting equitable partnerships between LMIC and UK institutions to address HPSR and capacity needs; including the roles of the Joint Lead Applicants and Co-applicants and plans for developing memoranda of understanding and collaboration agreements.
- Local Context: Please give a description of how you will review the relevant local context, existing research literature, and give an overview of the health systems landscape.
- Needs analysis and Stakeholder Engagement: Please outline your methods and approach to develop a needs analysis,torefineODA-eligible research questions and priorities through engagement with policy makers, evidence users and local communities, as appropriate. For example to consider:
- What HPSR research priority / need area will be explored in partnership with LMICs and with which LMICs (country and institution).
- How the planned approach fits within a health policy and systems research / health policy agenda.
- how you intend to identify and work with policy-makers, evidence users, stakeholders.
- How you will ensure LMIC institutions take a lead and outputs will be used.
- Capacity and Capability: Please outline your approach to establishing plans for developing institutional and individual capacity and capability particularly in lower resourced settings, for example:
- undertaking a needs assessment for capacity building (can be at institutional, national, regional level).
- ensuring the team and partnerships incorporate the range of disciplines and experience necessary to carry out the study and maximise existing expertise.
- developing research career development programmes and training; exchanges with policy-making institutions/practice-based settings; and capacity building in cross-cutting areas, such as grant management, finance management, contracting.
- Dissemination and Impact: Please provide an outline of the plans for ensuring impact and evaluating the outcomes, research uptake and dissemination.
Supporting further research programmes (1000 characters):
The purpose of this section is for the applicants to describe the planned outputs of the research and to provide details as to how they will ensure the outputs from the Development Award would lead to and/or contribute to a further research programme which would be submitted to a global health research funder.
Existing partnerships and ongoing work (2000 characters):
Please use this section to outline details of:
- Any relationships that already exist between the institutions/countries, the Joint Lead Applicants and Co-Applicants.
- Any existing partnerships or ongoing work that is already underway or planned.
- Any work that is being undertaken by other projects. How will your study impact on existing research projects and in country resources.
ODA compliance (2500 characters):
Please provide an ODA compliance statement which should explicitly demonstrate how the proposal meets key ODA requirements. It must answer the following questions in order:
- which LMIC(s) on the Organisation for Economic Cooperation and Development’s (OECD) Development Assistance Committee (DAC) list of ODA-eligible countries will directly benefit;
- how the application is directly and primarily relevant to the development challenges of those countries; and
- how the outcomes will promote the health and welfare of people in the country or countries on the DAC list.
- Where partner countries are listed as middle-income on the DAC list (for example China, India, Brazil), the application should demonstrate how the research will benefit the health and welfare of the poorest and/or most vulnerable groups of people in those countries and how their findings could have wider applicability to other low income countries.
Community engagement and involvement (2000 characters):
Community engagement is at the heart of NIHR so we would like to hear more about the practicalities of how you will plan for appropriate and relevant community engagement and involvement and describe how LMIC partners will be engaged to lead the priority setting activities to support the aims of the research plan.
Please note: your answer should not duplicate/repeat the information given in your ‘summary of activities to be undertaken (heading)’.
Consider the following and give a clear description of your approach:
- What is the purpose of community engagement and involvement in your plans?
- How you plan to identify relevant communities and organisations as potential partners from ODA-eligible countries.
- How you plan to establish effective ways of communicating.
- How you plan to support and enable relevant community groups and organisations to contribute to research as partners.
- What has changed or been included as a result of community engagement and involvement?
Contact information
The NIHR systems and the contract require the administrative ‘lead applicant’ to be from a UK based institution.
Please complete the UK Joint Lead applicant’s name, contact details and other requested information.
ORCiD
Please note: It is NIHR policy that all applicants obtain a free unique ORCiD number and update their MIS user profile to include their ORCiD before the application can be submitted. By clicking the link ‘View ORCiD record’ you will be taken to the ORCiD website where you will need to register or sign in. Once logged in to ORCiD and following acceptance of T&Cs, you will be returned to the MIS and the profile field for your ORCiD number will automatically be populated. You will only have to do this once. This is a mandatory requirement.
Research team
Definitions and requirements:
Joint Lead Applicants: It is a requirement to have two Joint Lead Applicants with one being from a DAC list Country and one from a UK-based institution. Joint Leadership must be demonstrated with plans in place for equal sharing of responsibilities. The applications will be judged on the equity and strength of lead applicants and the proposed development of the partnership.
Co-applicants: Those individuals with responsibility for the day to day management and delivery of the project. Co-applicants are considered part of the project team and are expected to share responsibility for its successful delivery. Please note that once you enter a co-applicant’s details they will receive an automated email informing them that this information has been added into our Management Information System (MIS) in conjunction with your application. Therefore, we would expect co-applicants to have been consulted before adding their details into the MIS.
Collaborators: Those who normally provide specific expertise on particular aspects of the project but who do not share in the responsibility for the delivery of the project.
Other supporting roles: As a minimum the following (mandatory) UK, based supporting roles are required to be added to an application:
- Administrative Authority or Finance Officer
- Head of Department
ORCiD ID
Please note: It is NIHR policy that all applicants obtain a free unique ORCiD ID number and update their MIS user profile to include their ORCiD ID before the application can be submitted. By clicking the link ‘View ORCiD record’ you will be taken to the ORCiD website where you will need to register or sign in. Once logged in to ORCiD and following acceptance of T&Cs, you will be returned to the MIS and the profile field for your ORCiD number will automatically be populated. You will only have to do this once. This is a mandatory requirement.
Joint Lead Applicants:
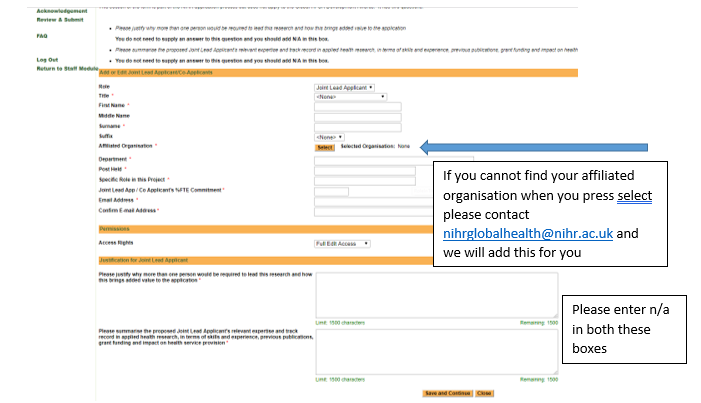
Add details of the Joint Lead Applicant as requested. Please note that once you enter the Joint Lead Applicant’s details they will receive an automated email informing them that this information has been added into our Management Information System (MIS) in conjunction with your application. Therefore, we would expect you to have consulted with the Joint Lead Applicant before adding their details into the MIS. Please ensure you use the correct email address for your Joint Lead Applicant if they are already registered on MIS.
Please note: you may not find your Joint Lead Applicants affiliated organisation in the current list. If you do not please contact the Global Health team at nihrglobalhealth@nihr.ac.uk who will add this for you.
Permissions: The access rights should be set to ‘Full Edit Access’ if you wish the Joint Lead applicants to be able to edit the application.
Justifications for Joint Lead Applicant:
This section of the form is part of the NIHR application process but does not apply to the Global HPSR Development Awards. It has two questions:
- Please justify why more than one person would be required to lead this research and how this brings added value to the application.
You do not need to supply an answer to this question and you should add N/A in this box. - Please summarise the proposed Joint Lead Applicant’s relevant expertise and track record in applied health research, in terms of skills and experience, previous publications, grant funding and impact on health service provision (1500 characters)
- You do not need to supply an answer to this question and you should add N/A in this box.Please note: The justifications for Joint Lead Applicant section will not be visible in your final application.
Once the Joint Lead Applicant has been added you must press ‘notify’. The following will then happen:
- The Joint Lead Applicant will receive an email from the MIS notifying them they have been invited to participate in the GHPSR application.
- The Joint Lead will be required to log into the MIS and click on the ‘Accept GHPSR Application Participation (Joint Lead Applicant)’ task. To do this, they must agree to the carbon reduction guidelines and choose ‘agree to participate’ from the drop down menu.
- The Joint Lead Applicant must then press submit.
- Once the Joint Lead Applicant has ‘agreed to participate’ they will be given two tasks:
- Collaborate on GHPSR Application (Joint Lead Applicant). This task requires completion and submission before the application can be submitted. You should ensure the Joint Lead Application is aware of this requirement.
- Global HPSR Application. This task gives the Joint Lead Applicant access to the application content and provided you have given ‘Full Edit Access’ they will be able to edit the application form.
Co-Applicants:
Add details of all co-applicants and their specific role in the project if applicable; however, it is not a requirement of the NIHR Global HPSR Development Award to have co-applicants. The number of co-applicants is calculated automatically.
Permissions: The access rights should be set to ‘Full Edit Access’ if you wish the Joint Lead applicants to be able to edit the application.
Please note: Do not include collaborators in this section, they should be mentioned (if necessary) in the summary of activities section of the on-line application form.
Other supporting roles:
As a minimum, the following (mandatory) UK administrating institution supporting roles are required to be added to an application:
- Administrative Authority or Finance Officer
- Head of Department
- Please note: If, as UK Administrative Lead Applicant, you are also signing as Head of Department, you should not complete this signatory task until you are ready to submit your application form. Once the UK administrative lead applicant completes the Head of Department signatory task, various fields within the application form will become non-editable.
- In addition, other listed supporting roles should be added as necessary. At the time of adding the necessary supporting roles required to approve your application you are advised to inform them. Please note this will not apply to all proposals.Permissions: The access rights should be set to ‘Full Edit Access’ if you wish the Joint Lead applicants to be able to edit the application.
Electronic signatures:
Each person nominated to a supporting role will be required to tick a check box indicating that they have read and understood the terms on which they have been nominated for this proposal and accept this role. Ticking this box constitutes an electronic signature of the supporting role with regard to this application.
Once the application form is completed and prior to submission, the UK Administrative Joint Lead Applicant is also required to tick a check box to indicate that they have read and understood the terms on which they have been nominated as Joint Lead Applicant (UK administrative lead) for this proposal along with the associated documentation and therefore accept this role.
No original (wet ink) signatures are required for this application.
Signatory statements
Please ensure that the required signatories (above) are aware of the statements of responsibility that they are agreeing to by making an electronic signature.
Uploads
Please note that the funding committee will not consider any additional, non-requested documents during its review.
Required uploads:
Upload 1: Budget and Justification
Please upload your completed budget spreadsheet (excel template is available in MIS), which should include justification for all costs and assessment of value for money. You should refer to the separate finance guidance when completing your spreadsheet.
Upload 2: Lead applicants’ CVs
Please provide a 2-page CV for each of the Joint Lead Applicants (LMIC and UK), this should include the top 10 most relevant publications.
Upload 3: Letters of support
You should use this upload type to provide evidence of support from both the Joint Lead institutions. This should be in the form of a letter (one per institution) and it must give clear agreement of the institutions intention to participate in the study and their agreement to supply space, facilities and the time required for the person to carry out the work activities outlined in the application.
If you have included Co-Applicants in your application, they will also require a letter of support from their institutions.
Upload 4: References
Please list all references cited in the application, using either the Vancouver or Harvard referencing conventions. This will be limited to 2 pages.
PLEASE NOTE: The MIS will limit each upload to the specified number of pages and you will not be able to upload a larger document.
Acknowledgment
Agreement to the Terms and Conditions:
Please tick to state that you agree with the acceptance statement and the terms and conditions
Conflict checks:
Please declare any conflicts or potential conflicts of interest that you or your co-applicants may have in undertaking this research, including any relevant, non-personal and commercial interest that could be perceived as a conflict of interest.
Potential conflicts of interest exist when a relevant or secondary interest which if not declared may lead to a perception of bias, embarrass, or put the credibility of NIHR, the programme or the individual at risk.
Potential Conflicts of interest:
Personal financial: This includes where an applicant, their partner or close family member, have a financial or commercial interest in the research through other employment, honoraria, contracts, academic collaborations where income has been personally received or attributed, consultancies, directorships, shares.
Personal non-financial: This includes where an applicant, their partner or close family member have a non-financial or unremunerated involvement with organisations, such as directorships of companies or organisations honorary contracts, unpaid academic collaborations, memberships, charities, Trustees, membership of political or pressure groups.
Non-personal financial: This includes funding to a department or research institute and not to an individual.
Other Interests: Other interests not mentioned above, but which you consider could be perceived to lead to a conflict of interest.
Review and submit
Once the validation status of your application contains no red crosses, you will be able to submit. You will not see a submit button until that time.
Contact details
For enquiries about this call, please e-mail us at nihrglobalhealth@nihr.ac.uk or call +44 (0)23 8059 1859