NIHR's mission is to improve the health and wealth of the nation through research in health and social care. To benefit the NHS, public health and social care, research we fund needs to be widely applicable. And this means the people involved in health and care research need to be diverse.
Introduction
Randomised controlled trials (RCTs) are one way researchers can test their hypotheses, and trial new treatments or interventions. RCTs recruit a range of participants, and randomly assign those participants to groups. For example, some will receive the drug, intervention or treatment being tested, others will receive an inactive substance with no medicine. Participants in the group who receive the treatment are then compared to the group who did not receive the treatment.
Who participates in RCTs is of the utmost importance. We need a diverse range of participants, to ensure that the results of RCTs do not leave out any group and that interventions are generalisable to the population as a whole. The NIHR is the first UK-based funder to assess the diversity of RCT participants.
This report is the step in better understanding who participates in research. The data analysed in this report relates to 148 RCTs that published findings in the NIHR Journals Library (NJL) between April 2019 and March 2021 from clinical studies commencing between 2007 and 2017. These published reports provided the source for our data. During this 2 year period, whilst other studies were published in NJL, all RCTs published during this time have been counted among the 148. The results of this project and our analysis provide a snapshot of how diverse RCT participants were during this period. However, this period predates NIHR’s focus on equality, diversity and inclusion (EDI) and indeed the focus on EDI of many health and care research funders. We acknowledge that since this time period the environment and society itself have changed.
In 2021, we published Best Research for Best Health: The Next Chapter, highlighting inclusion as one of our key operating principles. Since then, NIHR’s focus on EDI has accelerated, and we published our first EDI Strategy 2022-2027 in September 2022. As part of our commitment to EDI, the diversity of RCT participants and the development of initiatives to include under-represented groups and under-served communities, is something we need to focus upon. As this report shows, when we compare RCT participants to the results of the 2011 census in England and Wales, we are broadly in line with diversity in the UK. We are committed to doing more in terms of improving representation in research, with a view to ensuring that health and care research is inclusive and applies to all members of society.
In the future we intend to evolve our approach to data collection and analysis. We are also committed to strengthening our relationships with other funders, patients, service users, carers and communities, and to ensuring that our research is carried out by diverse teams. There is already work underway in these areas. Our Be Part of Research service provides information to the public about current research, and getting involved. Our Patient and Public Involvement and Engagement (PPIE) teams and our regional hubs promote community links, and support researchers to build relationships across all areas of the country, and beyond. This project is an important and positive step in our journey towards becoming a more inclusive funder of research.
Methodology
Data in this report was drawn from randomised clinical trial results published in the NIHR Journals Library (NJL) over a two year period.The RCTs took place prior to 2019, and relate to research projects which began between 2007 and 2017. NJL has 5 journals which correspond directly to 5 of NIHR’s research programmes. These are:
- Efficacy and Mechanism Evaluation
- Health and Social Care Delivery Research
- Health Technology Assessment
- Programme Grants for Applied Research
- Public Health Research
Focusing on papers published between March 2019 and March 2021, 148 RCTs were initially selected for analysis. Some of these papers did not publish data about participants, or were analyses of previously conducted RCTs and thus were excluded from this project. This meant that 140 RCTs fell within the scope of this project.
We have conducted analysis of three protected characteristics across these identified RCTs. Data was taken from the baseline characteristic tables accompanying published research findings. Participant data in baseline characteristic tables is completely anonymous and relates to the total number of participants in an RCT, rather than the characteristics of any individual participant. A typical example is shown in Table 1.
NJL follows recognised guidance for reporting the results of RCTs, with all published research being required to follow this guidance. However data was not collected as part of an EDI related data collection. Therefore, there is some variation in the number of reports for each of the three protected characteristics.
Table 1 - Example of baseline characteristics. (Source: Humby F, et al. Rituximab versus tocilizumab and B-cell status in TNF-alpha inadequate-responder rheumatoid arthritis patients:the R4-RA RCT. Efficacy Mech Eval 2022;9(7)
| Patient characteristics (Pathotype, n (%)) | Total (N=161) | Treatment group Rituximab (N=82) | Treatment group Tocilizumab (N=79) |
|---|---|---|---|
| B-cell poor | 79 (49) | 38 (46) | 41 (52) |
| B-cell rich | 64 (40) | 33 (40) | 31 (39) |
| GC* | 9 (6) | 5 (6) | 4 (5) |
| Unknown | 9 (6) | 6 (7) | 3 (4) |
| Sex (female), n(%) | 128 (80) | 62 (76) | 66 (84) |
| Age (years), median (IQR) | 55.5 (47.4-65.3) | 55.7 (47.7-65.5) | 55.5 (47.3-65.1) |
| Disease duration (years), median (IQR) | 9 (4.0-19.0) | 10 (4.0-20.7) | 9 (4.0-18.0) |
Where possible we have analysed RCT participants’ diversity by age, sex and ethnicity. In our Diversity Data Report we currently publish data on disability for our funding applicants, award holders, and committee and panel members. Our initial aim had been to also capture data relating to disability for participants in NIHR studies within the scope of this work. However, none of the 148 RCTs provided data in a format that clearly indicated how many participants declared a disability.
Many of the baseline characteristic tables included data on participant or patient symptoms. To make judgement about which of these constitute a disability would have been based on assumptions and not from a participant’s own declaration. As such, we have not provided an analysis of the disability of participants.
We collected data for each of the three protected characteristics as follows:
Age
85.7% of RCTs provided data relating to participants’ age in the baseline characteristic table. However in the vast majority of papers, participants’ age provided in the baseline characteristics table was presented as a mean. As such, we were unable to conduct analysis on anything other than mean age of participants, or to provide an age ‘grouping’ or ‘banding’ breakdown.
Sex/Gender
90% of RCTs reported on sex/gender. All responses extracted from the RCTs reported male/female data, and so we have reported on participant sex. However we recognise that there is conflation of sex and gender in some of the data collection, so the data we present may not relate exclusively to sex. There were no RCTs reporting numbers of trans and/or non-binary participants in the baseline characteristic tables.
We exclude single-sex trials from overall analysis. Single sex trials allow researches to focus either on a specific test population to analyse differences in effectiveness of a treatment on a particular sex, or to focus on a condition or biological process which might only affect a particular sex. Excluding these from overall analysis ensures the findings in relation to sex are applicable to all mixed-sex trials, and that we present an accurate picture of the RCT population.
Ethnicity
60% of RCTs provided a breakdown of the ethnicity of participants. Across the 148 studies that we have included in our data analysis, there were a total of 68 distinct ethnicity ‘categories’ recorded. Reporting at this level of granularity would not allow for meaningful comparisons with ONS data. There were also overlaps between the ways in which ethnicity categories were recorded across the published participant tables, so we have aggregated these categories into ‘Black’, ‘Asian’, ‘Mixed’, and ‘Other’. Appendix 1 shows the way we have aggregated these categories.
Prefer not to say (PNTS)
Responses were recorded variably, with some studies recording the number of participants with a PNTS response, and others recording ‘missing’ or ‘unavailable’ data. Where this information is available, it has been included in the graphs.
How the data has been analysed
Our approach in analysing the data available from the NJL data collection exercise has been as follows:
- Age: We present the mean and range age data.
- Sex: We exclude single-sex trials from overall analysis. The section of this report which focuses on sex highlights how many single-sex trials there were in total, and how these were split in terms of male and female focused RCTs.
- Ethnicity: Ethnicity data has been aggregated as discussed above. Our analysis of ethnicity relates to the entire RCT participant population.
- Comparator data: Where possible, we have given comparator data using the ONS census 2011 results. This is to assess how representative NIHR’s RCT participants are of the population of England and Wales as a whole.
Considerations for interpretation of data in this report
This project was a pilot, to test whether collecting RCT participant diversity data from published baseline characteristic tables was possible. NIHR did not give guidance to researchers relating to collecting diversity data. Because of this, there are differences in the number of studies who reported age, sex, and ethnicity in their baseline characteristic tables.
We have provided the percentage of studies that included data on each of the three protected characteristics. Our findings therefore only relate to the studies including data on each protected characteristic, and may not represent the diversity of participants in studies which did not include that data. We have not made assumptions about the studies that did not include particular data in the baseline characteristic table.
We have chosen the results of the 2011 census as a comparator to allow us to benchmark the diversity of participants against the diversity of the populations of England and Wales. As many of the RCTs took place around this time, the 2011 census is an appropriately timed comparator dataset.
As age data was reported most often as ‘mean age’ we do not have a list of individual participant ages or age groups from which to conduct analysis. As census data uses age grouping or median age, it is not possible to compare with our mean age data in a meaningful way. As such, we have not benchmarked analysis relating to age.
Report highlights
- The mean ages of RCT participants ranged from 2 to 86 years old. This range is expected, given the need to trial health initiatives, interventions and treatments across the whole population. There were also neonatology trials funded by the NIHR, but these data have been handled separately as the ages were typically provided in a different format, such as gestational weeks of age.
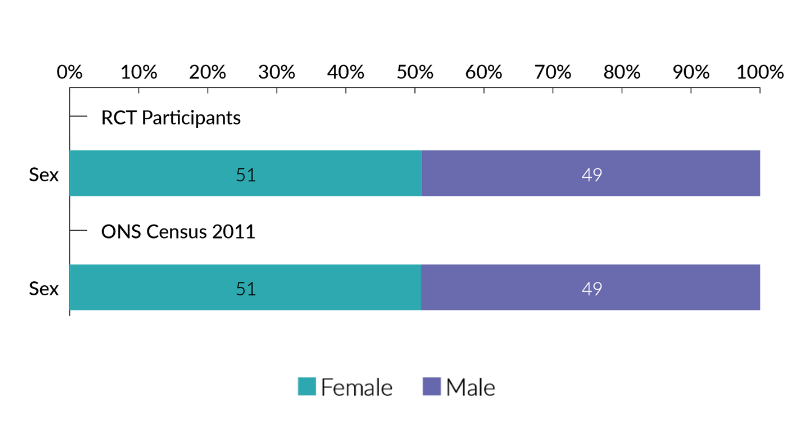
- In terms of sex, when assessed across all RCTs, male and female participation was broadly equal, at 49% and 51%, respectively.
- Single-sex trials were excluded from overall analysis of sex. Of the 140 RCTs in scope, there were 14 female only trials, and 4 male only trials.
- 86% of RCT participants were white. Black participants comprised 4% of RCT participants. A further 5% of participants were Asian. These figures are broadly in line with the 2011 census data on ethnicity across England and Wales.
Randomised clinical trial participant diversity
The following sections provide a breakdown of the results of our analysis for age, sex, and ethnicity.
Age
- The overall mean age of RCT participants across all RCTs analysed was 51.
- The range of participants' mean age was 84 years. Mean ages of participants ranged from 2-86.
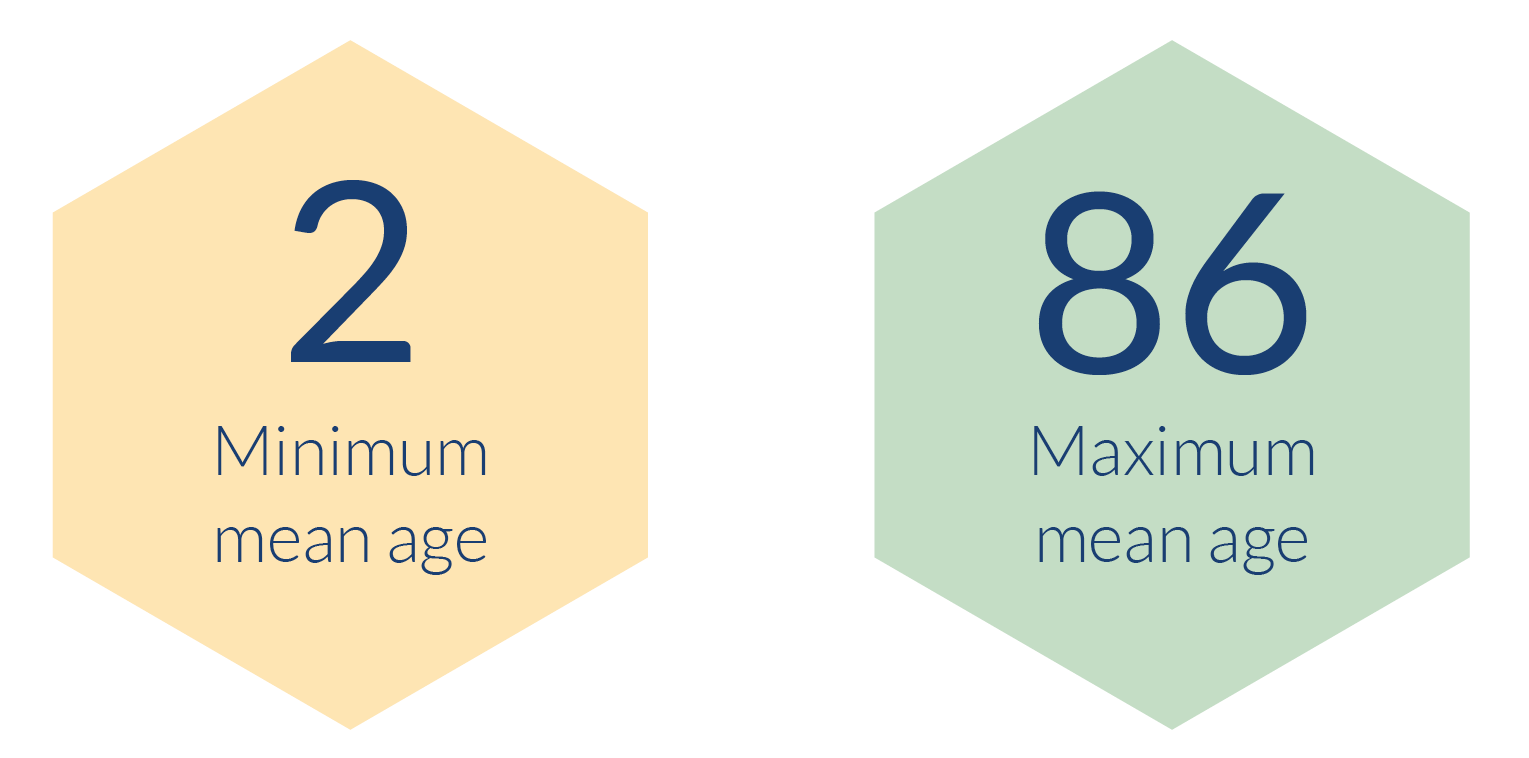
As noted in the methodology section of this report, we are unable to compare our analysis of age with the 2011 census. The census uses age bands and median age. We are reporting the mean age of participants as this is what was provided in the majority of the RCTs which reported on the age of participants.
Sex
- Across RCT populations as a whole, a broadly equal split of male and female participants took part in trials.
- 49% of trial participants were male, 51% female.
- The number of prefer not to say responses, or missing information about participants’ sex was minimal at less than 0.1%.
- There were 18 trials who recruited participants of a single sex. To ensure single-sex trials did not give inaccurate perceptions of the total trial population, we have excluded single-sex only trials from analysis.
- There were 14 single-sex trials who recruited female participants only, and 4 single-sex trials who recruited only males.

As noted in the methods section, we have conducted our analysis based upon sex. It is possible however, that non-binary and trans individuals who met the participation criteria may have participated in trials we have categorised as single-sex.
Total RCT participants by sex (excluding single-sex trials)
Across all of the NIHR RCTs which fell into the scope of this project, 90% provided information on the sex and/or gender of participants. As single-sex trials were excluded to conduct the below analysis, the findings below relate to 77% of RCTs in scope.
Our trial participants across all programmes and RCTs combined, when analysed by sex were almost identical to the results of the 2011 census. There were slightly more females than males who took part in RCTs, however this is reflective of the slightly higher proportion of females than males in the population. <0.002% of responses were PNTS. This is such a small percentage that it is not reflected in chart 1.
Chart 1 - RCT Participants by sex (excluding single-sex RCTs) and comparison with ONS 2011 census (Source: Population Estimates for the United Kingdom, March 2011)
Ethnicity
- Across all RCTs, 13% of participants were from a Black, Asian, Mixed Ethnicity, or ‘Other’ Ethnic minority background.
- census data from 2011 indicates that NIHR’s RCT population was broadly representative of the population of England and Wales.
- 86% of RCT participants were white. This is the same percentage of individuals from white ethnic backgrounds as was reported in the 2011 census.
- Ethnicity had a higher percentage of ‘prefer not to say’ responses reported, or data which was unknown or unavailable.
60% of the RCTs analysed provided data relating to the ethnicity of trial participants. We did not analyse ethnicity by programme. This is because some programmes have extremely small numbers of RCTs whose baseline characteristic tables include ethnicity data. The analysis would therefore not provide a useful insight into the diversity of different programmes.
Chart 2: RCT participants by ethnicity
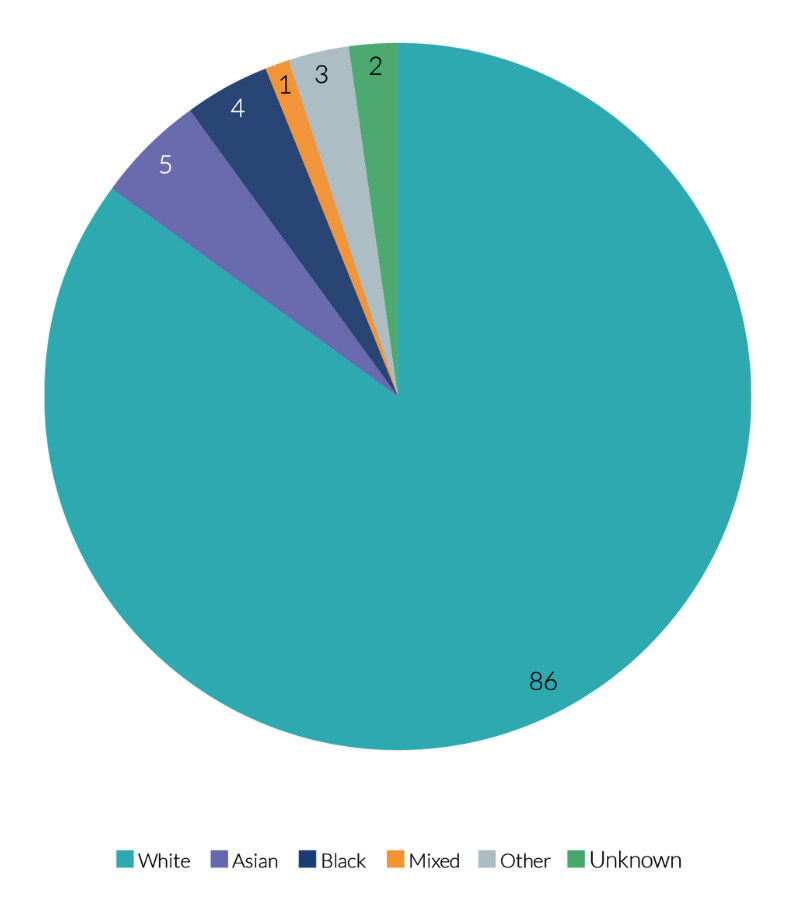
Chart 2 shows the proportions of RCT participants from different ethnicities, compared to the 2011 census results. This tells us that, based on the 2011 census, NIHR’s RCT is not hugely dissimilar to the diversity of the 2011 census population. While the RCT population has a smaller proportion of Asian individuals than the census population, we have a slightly higher proportion of Black individuals, and individuals from other ethnic backgrounds who participated in RCTs.
Chart 3: Analysis of NIHR’s RCT population compared to 2011 census population (Source: Ethnicity and National Identity in England and Wales 2011)
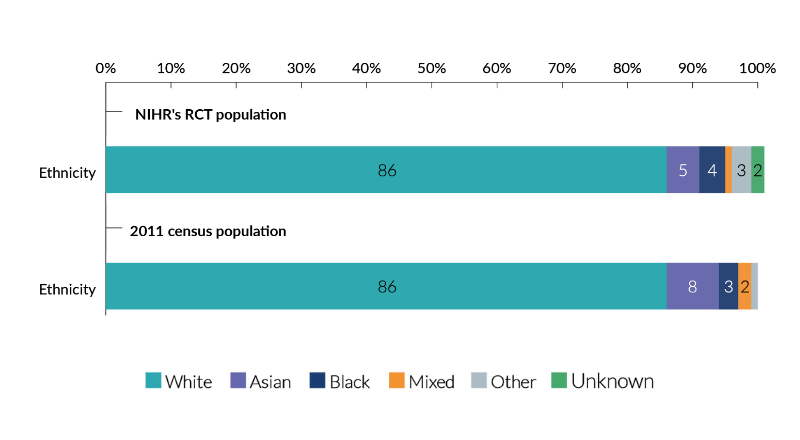
As chart 3 illustrates, the ethnicity of our RCT participants was broadly in line with the diversity within the population. There is, however, a significant percentage of the RCT population whose ethnicity is not included in the analysis. This is because only 60% of baseline characteristic tables provided data relating to the ethnicity of participants. This is an area where further work on data collection is needed.
Future steps
This analysis is the result of a pilot project. Whilst the findings of our analysis are reassuring, we know that there is much more work required to better monitor, and improve RCT participant diversity.
The list is not exhaustive, but details some of the focus areas for future work:
- To ensure that the diversity data we have is consistent across RCTs, we will provide guidance to Clinical Trials Units regarding the collection and sharing of diversity data.
- Following good practice and government guidance, we will provide a question and answer set that researchers are able to use when collecting diversity data. This question and answer set will be the same as that used across NIHR’s people framework.
- As outlined in the Women’s Health Strategy and as part of the MESSAGE project, and recognising health disparities, we will encourage research teams’ understanding of the differences between sex and gender and promote the need for disaggregating research findings by sex and gender.
- In collaboration with NHS Digital, we will collect data on the age, sex, ethnicity, geographic location and deprivation status of NIHR-funded research participants to understand whether there are any groups who are less well represented and to target activity to improve inclusivity where it is most needed.
Frequently asked questions
Why is there variation in the number of studies reporting each protected characteristic?
The studies we include in this report received no guidance from NIHR about how to collect diversity data. As noted in the introduction, all of the RCTs in the scope of this pilot project predate NIHR’s focus upon equality, diversity and inclusion. Because of this, and because the age, sex and ethnicity of participants in any particular RCT may not have been directly relevant to the focus of the RCT, research teams may or may not have collected this information. In the future, we will provide guidance to research teams relating to collecting and presenting diversity data covering all 9 protected characteristics, as set out in the Equality Act 2010.
How does NIHR know whether research teams asked participants about their sex, or whether they were asked about their gender?
In short, we do not know how equality monitoring questions were phrased to RCT participants. We decided to analyse participant sex in this report as all baseline characteristic tables recorded ‘male’ or ‘female’ as the response options. This was the case even for those studies where the patient characteristic was labelled ‘gender’.
The results of this analysis align with the results of the 2011 census. Does this mean there is no focus needed on diversifying RCT participants?
No. The results of the analysis in this report are reassuring, and show that research participants were recruited from a range of demographic groups. This range is broadly representative of the population included in the 2011 census. However, we know that there are many health disparities, for instance, that Black and Asian women disproportionately experience adverse health outcomes in pregnancy. In order to understand, and to address such health disparities, this might mean in some cases that a focus on recruiting a more diverse range of participants is required.
List of abbreviations and acronyms in this report
- DHSC - Department for Health and Social Care
- NJL - NIHR Journals Library
- ONS - Office for National Statistics
- PNTS - Prefer not to say
- PPIE - Patient and Public Involvement and Engagement
- RCT - Randomised Controlled Trial
Appendix 1 - Ethnicity Categories
| Aggregated category | Response options included |
|---|---|
| Asian | Asian/Asian British; Asian (British Asian or other); Indian, Pakistani or Bangladeshi; Asian (Indian/Pakistani/Bangladeshi/other); Asian or Asian Mixed; Asian; Asian: Indian; Indian; Asian: Pakistani; Pakistani; Asian: Bangladeshi; Bangladeshi; Asian, Asian Scottish or Asian British; Other Asian background; Other Asian (non-Chinese); South Asian; Chinese; East Asian; Chinese or other |
| Black | Black African/Black Caribbean/Black British; Black UK/African Caribbean; Black UK/African; Black or Black mixed; African; Black African; Black British; Black/Black British; Black (Black British or other); Black; Black Caribbean; Caribbean; African Caribbean; Black other; Black (Caribbean/African/Other); Black African/Afro Caribbean |
| Mixed | White and Black Caribbean; White and Black African; White Asian; Mixed/Multiple; Mixed; Mixed (Caribbean/African/Asian/Other); Mixed ethnicity; Mixed: white and Black Caribbean; Mixed: white and Asian; Other mixed background; Dual Heritage |
| Other | Hispanic; Other; Other (including Indian, Pakistani, other Asian background, Chinese and mixed background); Other ethnic group |
| White | White English, Scottish, Welsh or Irish; White/Caucasian; White British/Irish; White UK/Irish; White; White British; White British, European or other; Australian born (non aboriginal); White/White British; White Irish; White other; White British/European; White British |

