Diverse and innovative research approaches are needed to tackle complex health and social care challenges. NIHR Programme Grants for Applied Research (PGfAR) is seeking to increase the novelty and ambition of research designs awarded funding. We have funded a number of awards in recent competitions that have risen to the challenge, including this case study.
Programme Details
Title: RAPID and Efficient Eczema Trials (RAPID programme)
Lead Researchers: Professor Kim Thomas and Mrs Amanda Roberts
Institution: Nottingham University Hospitals NHS Trust
Funding: £2,492,655.00
Programme Start Date: 1 September 2022
Patient and clinical need
Eczema is an itchy, long-term skin condition. The main treatments are topical treatments such as moisturisers (emollients) and flare-control creams (e.g. topical corticosteroids and calcineurin inhibitors), but how best to use them is still uncertain. Inconsistent messages and confusion lead to poor treatment adherence. Questions about skincare, washing and how to use eczema treatments are a high priority for patients but are rarely the focus of large, high-quality randomised controlled trials (RCTs).
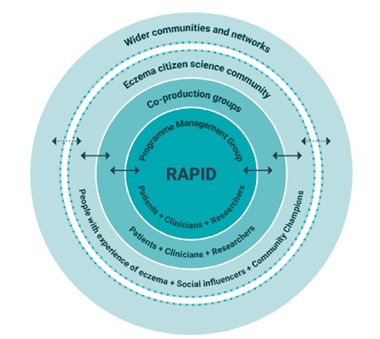
Thus, efficient and novel methodological approaches are required to address these patient priorities in a timely and resource-efficient manner, whilst providing robust evidence to inform management choices. This programme is developed with the aim of combining the strengths of collaborative Citizen Science (CS) with robust trial design to rapidly and efficiently answer research questions through a series of parallel-group online RCTs. This innovative approach ensures that trials can be conducted, and results implemented, much more quickly. For the purpose of this study, CS has been defined as a ‘scientific method of working with members of the public to define, address and share answers to questions that are important to them’. Citizen science democratises research, making it more relevant, accessible and inclusive, and is likely to improve uptake of study findings. Figure 1 below demonstrates the different groups used in this CS design; from the Programme Management Group, co-production groups, Eczema CS community and wider communities and networks.
Aims of the programme
This study aims to:
- Improve the lives of people living with eczema by partnering with ‘citizen scientists’ to deliver multiple, efficient online clinical trials.
- Share new knowledge that will empower patients and enhance knowledge about the management of eczema.
- Inform clinical practice and guidelines.
Programme design
By working with patients and members of the eczema community and following a co-production approach, the most important research questions will be prioritised. This will facilitate designing of feasible and inclusive interventions suitable for testing in large RCTs conducted online. New emerging evidence from these trials will be rapidly shared with the eczema citizen science community and new interventions added throughout the programme.
The research will be carried out through three interlinked workstreams (WS) in parallel with iterative learning from each informing delivery of the core activities.
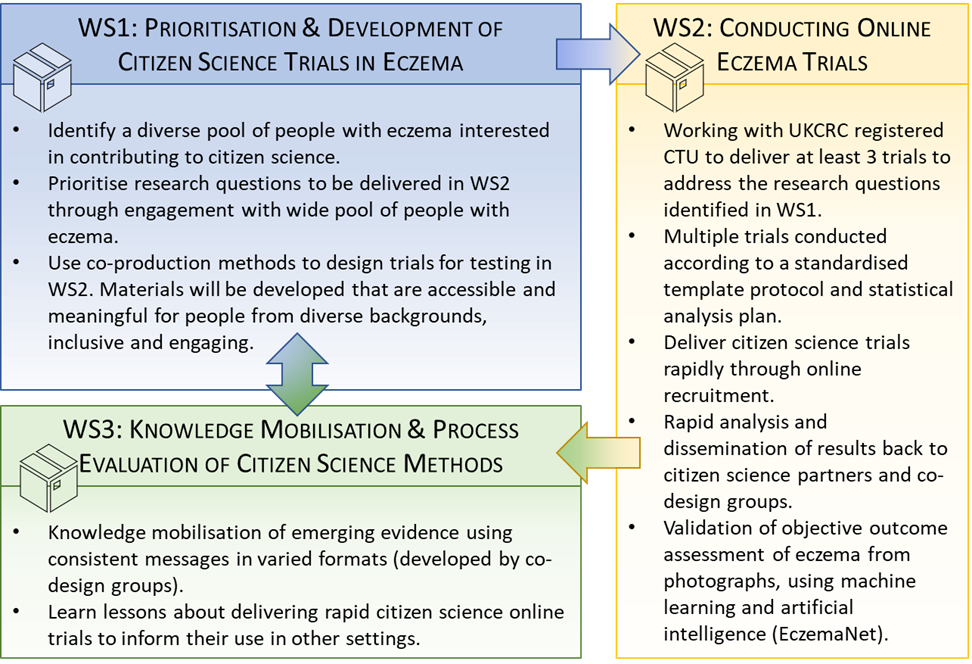
Workstream 1: Prioritisation and development of citizen science trials
Methods: The “Eczema Citizen Science Community”, a diverse pool of people with eczema willing to contribute to citizen science trials, will be established. In partnership with this community, research questions suitable for addressing in online trials (WS2) will be prioritised. Co-production methods will be used throughout the programme to prioritise, design and share the findings of the online trials. Materials will be developed that are accessible and meaningful for people from diverse backgrounds. Furthermore, trial-specific recruitment materials will be prepared that are inclusive and engaging.
Workstream 2: Conducting online eczema trials
Methods: At least three online randomised controlled trials (RCTs) will be conducted to test the interventions developed and address the research questions identified in WS1. The trials will be conducted according to a standardised template protocol and statistical analysis plan, and will be delivered rapidly through online recruitment. Results will be analysed rapidly and disseminated back to citizen science partners and co-design groups. Validation of an objective eczema severity assessment tool using artificial intelligence (EczemaNet) for evaluating digital photographs of the skin will be carried out. The feasibility and validity of using this approach in online trials, where physical assessment of the skin is not possible, will be evaluated.
Workstream 3: Knowledge mobilisation and process evaluation of Citizen Science methods
Methods: Co-production techniques using consistent messages in varied formats will be employed to accelerate knowledge mobilisation of the emerging evidence. Process evaluation methods will be designed to help identify transferable learning for delivering rapid citizen science online trials to inform their use in other settings, and to identify factors likely to facilitate implementation of effective interventions.
Why was this programme design chosen?
This programme will put the eczema community itself into the driving seat of high-quality research. The programme design will prioritise research questions that are genuinely important to this community, generate trust in marginalised groups, recruit faster and wider, and mobilise knowledge more meaningfully. This is expected to have advantages for both research waste, environmental considerations and will leave a long-lasting legacy to support others wanting to conduct efficient online trials.
Resources
For more information about this programme please visit the website and blog post.

