Clinical Research Facilities

NIHR’s 28 Clinical Research Facilities (CRFs) are purpose-built facilities in NHS hospitals where researchers can deliver early-phase and complex experimental studies. They offer a range of services to help researchers and life science organisations to design and deliver these clinical trials.
What are the NIHR Clinical Research Facilities?
NIHR CRFs are state-of-the-art, purpose built facilities based in NHS hospitals that are dedicated to delivering early phase, experimental medicine and high-risk studies. This includes first-in-patient trials and those that require dedicated space and specialist expertise.
The NIHR has invested £161 million in these facilities across England, creating cutting-edge facilities for complex studies, including overnight studies.
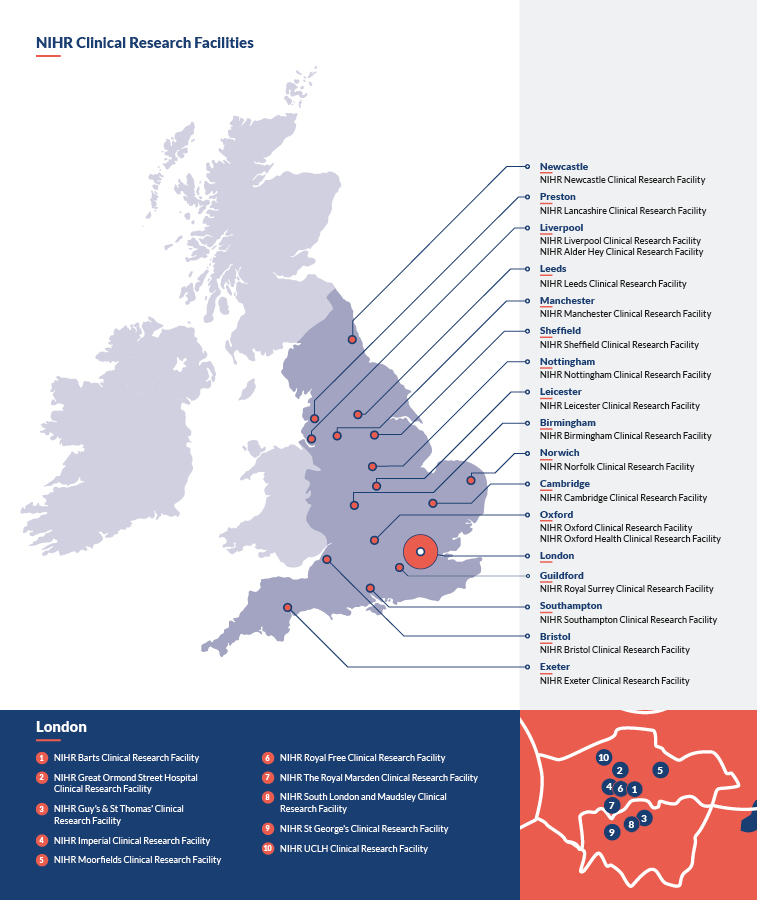
Where are the NIHR CRFs located?
Our 28 CRFs are distributed across England. These are:
- NIHR Alder Hey Clinical Research Facility
- NIHR Barts Clinical Research Facility
- NIHR Birmingham Clinical Research Facility
- NIHR Bristol Clinical Research Facility
- NIHR Cambridge Clinical Research Facility
- NIHR Exeter Clinical Research Facility
- NIHR Great Ormond Street Hospital Clinical Research Facility
- NIHR Guy’s and St Thomas’ Clinical Research Facility
- NIHR Imperial Clinical Research Facility
- NIHR Lancashire Clinical Research Facility
- NIHR Leeds Clinical Research Facility
- NIHR Leicester Clinical Research Facility
- NIHR Liverpool Clinical Research Facility
- NIHR Manchester Clinical Research Facility
- NIHR Moorfields Clinical Research Facility
- NIHR Newcastle Clinical Research Facility
- NIHR Norfolk Clinical Research Facility
- NIHR Nottingham Clinical Research Facility
- NIHR Oxford Clinical Research Facility
- NIHR Oxford Health Clinical Research Facility
- NIHR Royal Free Clinical Research Facility
- NIHR The Royal Marsden Clinical Research Facility
- NIHR Royal Surrey Clinical Research Facility
- NIHR Sheffield Clinical Research Facility
- NIHR Southampton Clinical Research Facility
- NIHR King's Clinical Research Facility
- NIHR St George's Clinical Research Facility
- NIHR UCLH Clinical Research Facility
All CRFs in England and the devolved administrations are supported by the UKCRF Network. The UKCRF Network is funded by the NIHR and it facilitates collaboration among CRFs, providing strategic leadership to create an internationally competitive network of early-phase facilities in the UK.
This networks enables:
- coordination of CRFs into a national early phase research delivery platform
- access to highly skilled research delivery staff
- development and sharing of best practice guidance and tools
- provision of support for patient involvement and access to early phase research
What services do the NIHR CRFs provide?
The CRF teams have a proven track record of helping to deliver a wide range of research. Researchers funded by the NIHR, the life sciences industry and other organisations can access assistance from skilled CRF clinical trial support staff, from study design to data collection and study management. This includes specialist world-leading expertise in specific therapeutic areas, highly specialised equipment and facilities, access to patient populations and patient and public advocates.
In 2022 to 2023, our CRFs collectively supported 1,774 active commercial studies.
How do CRFs support research?
CRFs receive sustained funding from the NIHR to deliver both commercial and non-commercial, early translational and experimental medicine research.
The CRFs can:
- provide state of the art facilities to support the delivery of high-intensity research,tailored to each CRF’s therapeutic specialisms such as laboratories for sample processing, clinical trials pharmacies, radiopharmacies, sleep clinics, cardiorespiratory exercise and hypertension testing suite, a pan-endoscopy suite, in-patient isolation rooms, and more
- offer a critical mass of dedicated and highly-skilled clinical staff, scientists and academics, who have specialist expertise in the delivery of experimental medicine and high intensity studies
- help capacity building through training and development opportunities
- provide opportunities to leverage and attract funding from external organisations, forming key strategic partnerships through industry engagement
- engage participants across a wide range of conditions, developing inclusive recruitment strategies to target specific patient populations
- connect and collaborate with networks of patients and volunteers who help in the design of patient-centric research
- offer access to project management and data collection expertise, and a diverse workforce of experience research nurses, to aid study delivery
CRF support for the life sciences industry
In addition to working with NHS and university-led research teams, CRFs have extensive experience of working with commercial companies, from biotechnology and medical technology startups to prominent pharmaceutical giants.
Companies that choose to work with NIHR CRFs can access all of the support for research listed above. This support is available to both commercial and non-commercial research studies.
Life science organisations and sponsors of commercial research should contact the NIHR industry team to discuss their requirements and explore which facility would be best suited to support them.
Visit our ‘Offer to the Life Sciences Industry’ page to discover the full range of support available to commercial research sponsors developing and delivering research in the UK.
