Patient Recruitment Centres

Patient Recruitment Centres (PRCs) are NIHR-funded research facilities dedicated to delivering late-phase and large-scale commercial clinical research in the NHS and wider health and care environment. Learn what we mean by ‘commercial’ research.
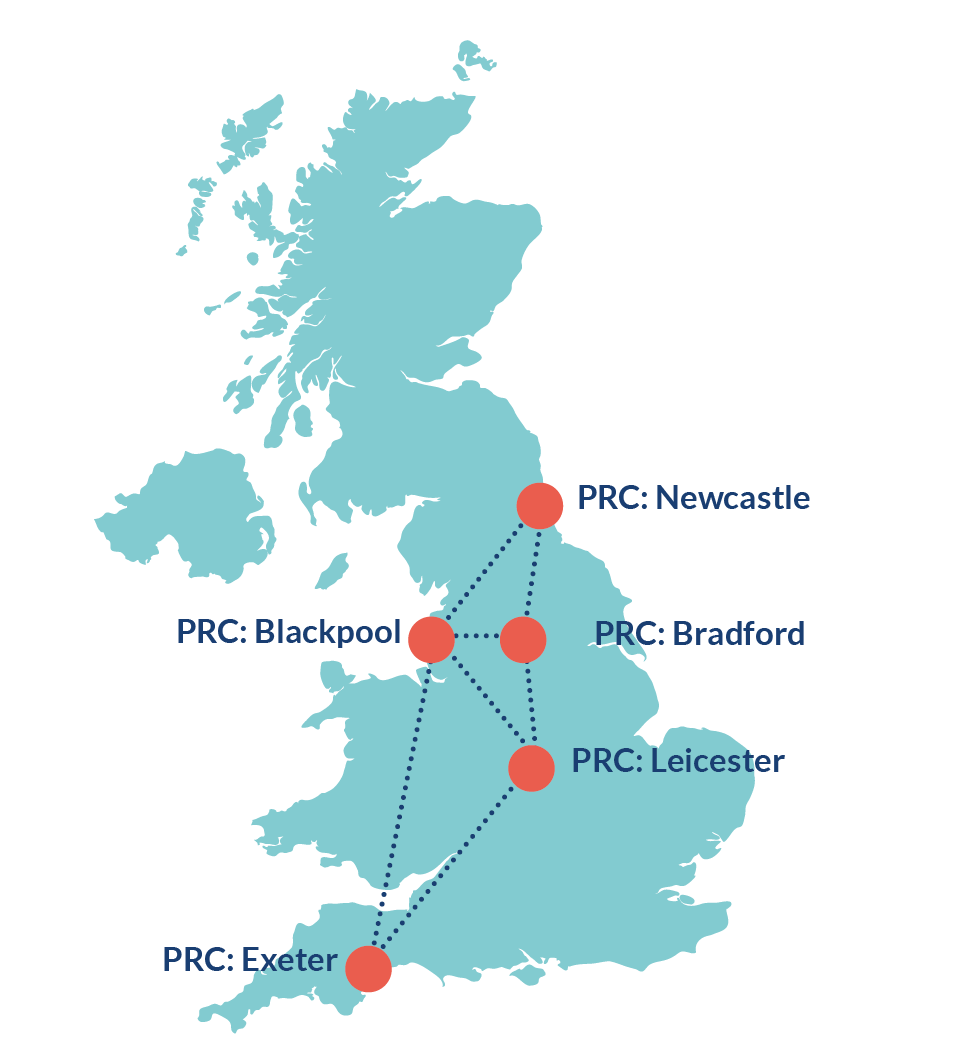
There are 5 PRCs in the UK.
Speed, efficiency, patient experience, innovation and continuous improvement are the key drivers of these five purpose-built centres which exclusively deliver commercial research at pace and scale.
Five reasons to place your research at our PRCs
This short video (under 3 minutes) highlights some of the stand-out features of the PRC offer to industry.
Key benefits of the Patient Recruitment Centres
With a special focus on common chronic conditions, these research facilities have a number of unique features.
Patient focused
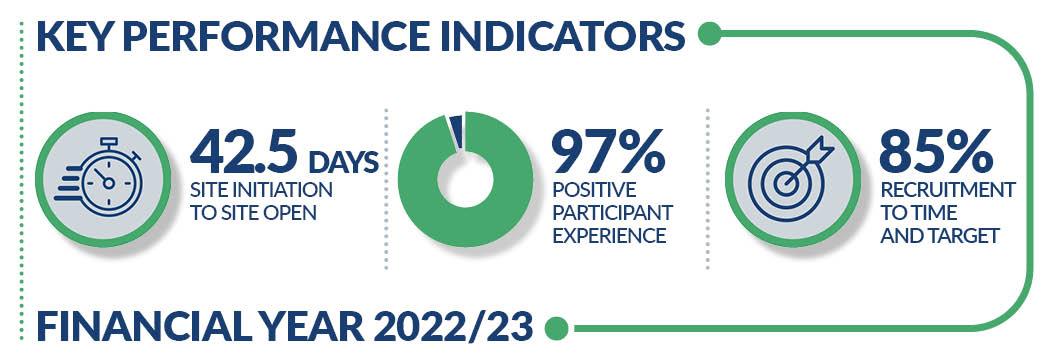
Our concierge-style service offers accessible hours of operation, welcoming waiting areas with free refreshments and parking for your participants. 97% of participants surveyed at our centres said they would take part in research again at a PRC.
Purpose-designed
Our state-of-the-art facilities are tailored for delivering late-phase, large-scale commercial research.
Collaborative
Our five connected sites may be geographically dispersed but our processes are streamlined with a single point of contact and consistent standard operating procedures.
Wider recruitment reach
We design proactive and inclusive recruitment strategies that can reach beyond specialist hospital clinics and access diverse patient populations in areas of high disease prevalence. For example, we have strong links to primary and community care for recruitment and remote monitoring.
Dedicated to commercial research
Our dedicated teams are experienced in working with sponsor companies and Contract Research Organisations and understand the demands of delivering commercial research.
Operational flexibility
We can rapidly scale up operations to meet individual trial requirements.
Innovative research delivery
We provide a test bed for innovation in clinical trial delivery including data-driven patient identification and engagement and pioneering trial capabilities, such as decentralised trial delivery.
Access to investigators
We have access to NHS clinical investigators to lead and conduct your research, across a wide range of therapeutic areas.
Our PRC sites
Want to know more? Why not take a look around our Patient Recruitment Centre web pages.
Further information about our PRCs
News: PRCs exceed targets for commercial research
Read how the PRCs have exceeded targets for set-up and recruitment to commercial studies
Case study: Excellent retention rates
Read a case study on how the PRCs achieved 95% retention rate in a Moderna vaccine trial
Case study: Decentralised delivery
Read a case study describing decentralised delivery of the Relieve IBS-D trial.
Case study: Rapid recruitment
Read a case study about the rapid delivery of the Novavax COVID-19 vaccine study.
Video: Participants’ perspective
Hear the patients' perspective as they share their experiences of taking part in trials at the PRCs.
Request a brochure
Request a copy of our PRC brochure (PDF) to learn more about what our five PRCs have to offer using the contact details below. Or read the plain text accessible version on our website.
Explore more support from the NIHR
This service is just one of the ways we help life science organisations. Visit our NIHR support for industry page to discover our full range of support or explore all our services and support.
Suggested content
Customers also viewed:
Contact the Industry team
Fill out our contact form to request a chat with our team. We aim to reply as soon as possible and always within two business days.
