Published: 05 August 2020
On 21 May 2020, the NIHR published a Framework to support the restarting of research paused due to COVID-19. Developed in partnership with multiple stakeholders and the devolved nations, the framework provides a flexible structure for local decision-making. Our goal is to restore a fully active portfolio of NIHR research while continuing to support important COVID-19 studies as part of the Government response to the pandemic.
Dr William van't Hoff, CEO of the NIHR Clinical Research Network (CRN) and Senior Responsible Officer for the Restart Programme, said: “Restarting research is happening alongside the restart and recovery of clinical services in the NHS and is proving complex and challenging. However, we know sites are making progress from the data and intelligence we are gathering through Local Clinical Research Networks, CRN national and local clinical leadership and our partnerships with UKRD and R&D Forum.
“We’re working closely with colleagues in UKRD and R&D Forum to provide support to sites by sharing experiences and solutions to common challenges in the restarting of research. This update is part of that effort.”
Key learnings
Key support departments can help smooth the restart process
The NIHR Imaging Group recommend that key support departments including radiology, pathology and pharmacy are included in local prioritisation panels. For example, having a radiology representative working alongside management and investigators to assess study viability will enable an informed judgement to be made as to what imaging work can be carried out safely, what may need to be either prioritised further or postponed, or require amendment, as well as establishing a single point of contact for such discussions - this will help coordinate and smooth the restart process.
Assessing the capacity of the research delivery workforce
We are very aware that sponsors and sites are concerned as to whether there is sufficient capacity in the research delivery workforce to support COVID-19 urgent public health studies, the restart of non-COVID-19 studies and the pending vaccine studies. We are working with the Department of Health and Social Care to assess the increase in demand and explore a number of options to address this.
The latest CRN data
CRN continues to improve its data collection, particularly in terms of commercial studies, and the following is our best currently available intelligence:
Based on data from 4,168 non-commercial studies, we know that 1,451 studies are open to recruitment. Of these, at least 742 have reopened to recruitment following a pause due to the pandemic.
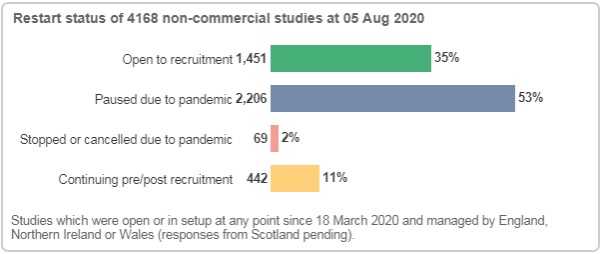
Following a new data collection exercise that gathers study-site intelligence from Local Portfolio Management Systems, the CRN can now report more granular information on each study site status. This data collection exercise also includes commercial recruitment data.
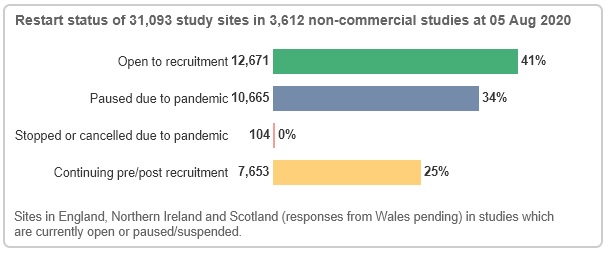
On the non-commercial side, data from 31,093 study sites indicate that 41% are open to recruitment and 34% are paused due to COVID-19. 25% are continuing pre/post recruitment - these are not currently in the recruitment stage of the study life cycle; they may be in set-up or, for reasons unrelated to the pandemic, suspended or recently closed.
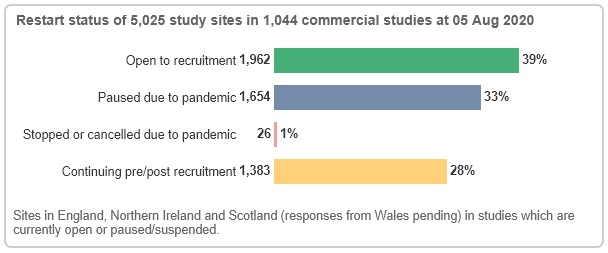
Similarly on the commercial side, data from 5,025 study sites indicate that 39% are open to recruitment, 33% are paused due to COVID-19 and 28% are continuing pre/post recruitment.
Restarting NIHR programme studies
Most NIHR programmes have been affected by the pandemic, but the impact has varied according to the type and complexity of the research. Very few projects are paused completely and a large number, particularly those that are not reliant on collecting new data from patients or service users, have been able to continue relatively unscathed. Those most strongly affected include complex programmes and trials where the disruption of NHS and social care services continues to pose challenges to recruitment, intervention delivery and data collection. Patient participants and other service users, working with researchers, are suggesting innovative and imaginative solutions, whilst being mindful of the need not to compromise the scientific integrity of the studies.
Whilst many teams are requesting funded extensions, most are for a relatively short duration (less than six months). Some are also requesting changes to their protocols, and others have indicated a wish to postpone their start dates until 2021. Such requests are being viewed sympathetically.
Encouragingly, only 22 projects across the entire portfolio are completely paused, and all lead researchers are being encouraged to scale up as far as possible. In a very small number of projects the lead researchers have indicated that their projects are no longer viable; most of these were in jeopardy prior to the pandemic. Although two thirds to three quarters have had elements of their projects affected, few are completely paused and most are now engaging positively with NIHR in the Restart process.
Clinical academics returning to research
At the outset of the COVID-19 pandemic more than 1,500 clinical academics returned to full-time frontline clinical roles, including the majority of people on the NIHR Integrated Academic Training pathway and HEE/NIHR Integrated Clinical Academic Programme. These researchers - and many others like them - put their own research, and careers, on hold to respond to the pandemic.
One NIHR Academy Member whose experience during the pandemic has been varied, challenging and rewarding is HEE/NIHR Clinical Doctoral Research Fellow, Anthony Gilbert. He has been able to use his existing research and expertise of virtual consultations to support a roll-out at his Trust and also increase his clinical practice. This included time at the NHS Nightingale London as Deputy Physiotherapy Lead. Read more of Anthony’s story.
In May, as part of the Clinical Academic Training Forum – a UK-wide group of funders – the NIHR published a series of principles to support clinical academics returning from the front line to their research roles. Most recently letters have been sent to NIHR Academy members to support them and their host institutions in the return of clinical academics to their roles by 5 August.
Professor Dave Jones, Dean for the NIHR Academy, said: “Over recent months we’ve seen large numbers of clinical academic trainees returning to full-time clinical duties to support the national response to COVID-19. We are all rightly proud of the contribution our researchers have made, and continue to make, in this area.
“It is therefore vital that we support our returning clinical academics and their host institutions in their research roles. The principles and actions we have shared will provide some initial guidance to facilitate this in an effective way.”
Previous updates:

