
Tests and diagnostics

Enabling a better and faster response
To control the spread of the coronavirus, accurate testing for COVID-19 is crucial. Our researchers are busy evaluating new tests and diagnostics to assess the accuracy of these tests, tracking the progress of those in development, and speeding up the pace at which they’re available to the health and care system.
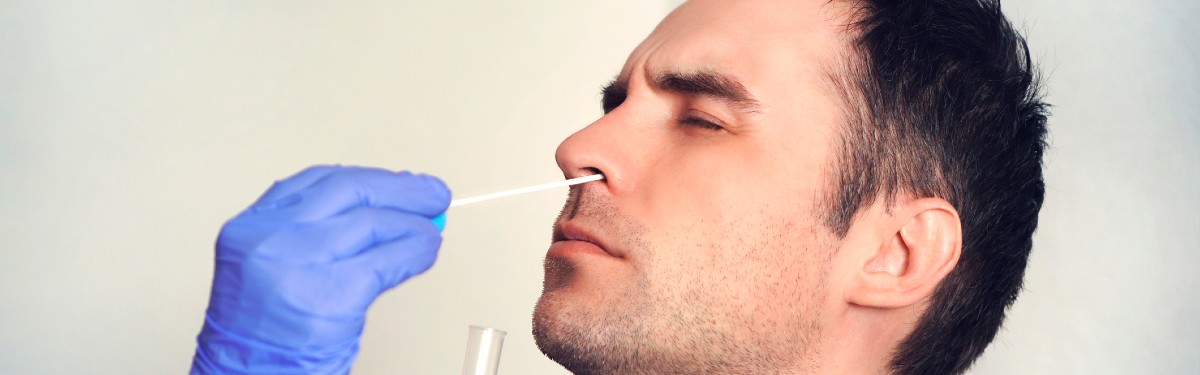
Testing the tests
The COVID-19 National DiagnOstic Research and Evaluation Platform (CONDOR), is speeding up the time it takes for COVID-19 tests to be ready to use. CONDOR is funded by the NIHR, in partnership with UK Research and Innovation, Asthma UK, and the British Lung Foundation. CONDOR tests new COVID-19 diagnostics in places such as GP surgeries, care homes or hospitals, as well as in laboratories.
This research has:
- led to the use of lateral flow tests for mass-testing in Liverpool, and of NHS staff
- shown the benefit of using a rapid 90-minute test in care homes
- shown the accuracy of a five-minute finger prick test
Collaborating to develop and assess diagnostics
NIHR Medtech and In vitro diagnostics Co-operatives (MICs) have been supporting our response to the pandemic, by:
- providing evidence on diagnostic tests
- evaluating new point-of-care tests
- responding to the need for diagnostic solutions for COVID-19
These MICs support researchers and the life sciences industry to develop new medical technologies, and provide evidence on commercially-produced in vitro diagnostic tests.
For example, the NIHR Cardiovascular MIC has supported the development of new techniques to assess lung disease, in patients referred for a cardiac MRI. These novel lung sequences have been included in guidance on the assessment of patients with COVID-19.
Horizon scanning
The NIHR Innovation Observatory (NIHRIO) tracks COVID-19 diagnostic tests that are currently in development, and commercialised worldwide.
NIHRIO sends a weekly update to national organisations such as NHS England and NHS Improvement, the Medicines and Healthcare products Regulatory Agency, and the Department of Health and Social Care. This helps them to assess priorities, identify tests with the potential to reduce testing pressures, and deal with other testing issues.


