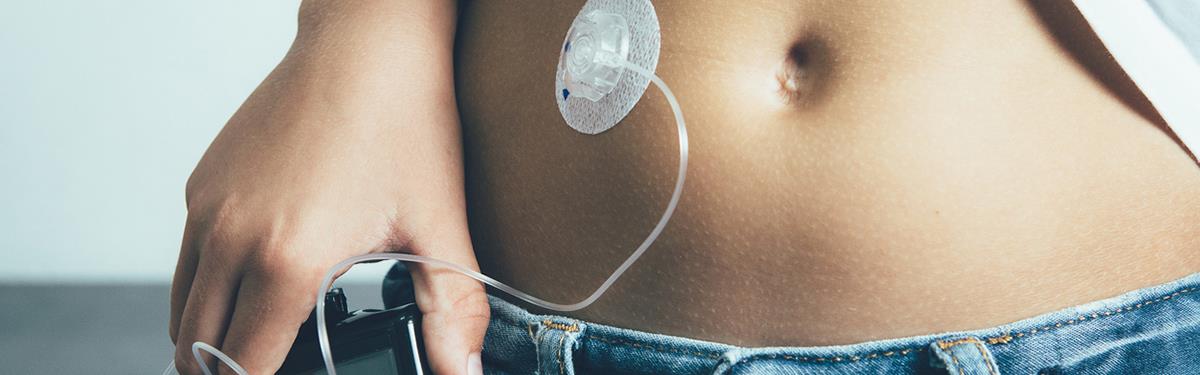
An artificial pancreas developed by NIHR-supported researchers and recommended by NICE is changing the lives of people with type 1 diabetes.
Published: 22 September 2023
The lifelong challenge of diabetes
More than 400,000 people in the UK live with type 1 diabetes and depend on insulin delivery through injections or an insulin pump to survive. Unlike type 2 diabetes, it cannot be prevented or controlled by lifestyle changes. Insulin is normally produced by the pancreas to control blood sugar (glucose) levels. Poor blood sugar control can lead to serious complications including blindness, heart disease and kidney problems.
For those with diabetes, the mainstays of blood sugar control are frequent finger-prick tests, insulin injections and, more recently, continuous glucose monitors and insulin pumps. However, many people struggle to manage their blood sugar levels this way and researchers are constantly trying to improve treatment options and patients’ quality of life.
Supported by the NIHR Cambridge Biomedical Research Centre (BRC) and NIHR Cambridge Clinical Research Facility (CRF)*, Professor Roman Hovorka led ground-breaking research on the “artificial pancreas” to provide insulin automatically. “Our aim is to alleviate the ever-present burden of type 1 diabetes and improve health outcomes for individuals with diabetes and their families,” said Professor Hovorka.
The artificial pancreas uses a glucose sensor under the skin to measure a person’s blood sugar levels. The researchers’ CamAPS FX mobile phone app receives these blood sugar levels wirelessly and automatically delivers insulin continuously by pump.
The artificial pancreas – the future for diabetes?
Over the last 15 years, the NIHR Cambridge BRC and CRF have supported the device’s development and extensive testing in over 30 separate studies. These have involved hundreds of patients**, including pregnant women and very young children in whom diabetes can be difficult to control.
Proper management of type 1 diabetes during pregnancy is crucial to avoid serious complications for both mothers and babies. In a much-needed breakthrough, a trial published in Diabetes Care showed that the artificial pancreas technology safely improved pregnant women’s blood sugar control.
In a separate study of 1 to 7-year-old children published in The New England Journal of Medicine, the device helped families manage their children’s blood sugar levels more effectively than with their usual, current technology. Any improvements are particularly important among children as their blood sugar levels can quickly rise or fall to dangerous levels. “We believe our artificial pancreas will transform the lives of families with very young children affected by type 1 diabetes,” said Professor Hovorka.
The numerous trials conducted at NIHR facilities paved the way for Professor Hovorka to set up a company, CamDiab, to commercialise the artificial pancreas. It is now publicly available in 15 countries including Germany, Poland and Australia.
This was followed in 2023 by NICE recommending the artificial pancreas technology for patients who struggle to control their type 1 diabetes. Its approval will benefit more than 100,000 people in England alone.
“The clinical and economic evidence delivered by randomised controlled trials at the NIHR Cambridge BRC and CRF were very pivotal during NICE’s review of the device and its subsequent recommendations for its use in the NHS.”
Roman Hovorka, Professor of Metabolic Technology, University of Cambridge
A safe and effective approach for type 2 diabetes
People with type 2 diabetes are also seeing the benefits of the new technology. Working with researchers from Switzerland, Professor Hovorka’s team trialled a modified version that automatically delivers insulin by pump without needing dietary information via an app. In studies published in Nature Medicine, this version was first used successfully by people living with both type 2 diabetes and kidney failure, and then among people with type 2 diabetes alone.
Together, this research has made significant progress towards making the new technology available to the 5 million people in the UK living with type 1 and 2 diabetes, while reducing the £10 billion annual cost of diabetes care to the NHS.
*The NIHR supports the translation of scientific developments into clinical treatments through its 20 BRCs. The BRCs operate as partnerships between local NHS organisations and academic institutions such as the University of Cambridge. The NIHR also supports 28 CRFs, which are dedicated spaces for delivering research and trials.
**The studies were funded by numerous organisations including Diabetes UK, Juvenile Diabetes Research Foundation and the European Commission.
More information about the studies is available on the NIHR’s Funding & Awards website.

