Biomedical Research Centres

NIHR’s 20 Biomedical Research Centres (BRCs) are networks of experts that work collaboratively between NHS trusts and internationally renowned universities. They facilitate early stage experimental medicine research and support the translation of scientific discoveries.
What are the NIHR Biomedical Research Centres?
NIHR Biomedical Research Centres (BRCs) bring together academics and clinicians to translate early scientific breakthroughs into potential new treatments, diagnostics and health technologies.
The NIHR has awarded nearly £800m in funding to facilities across England, creating an environment where experimental medicine can thrive.
BRCs attract the best scientists and produce world-leading research, improving patients’ lives, and contributing to the local and national economy. Their high-quality, innovative research also attracts significant investment from external funders, furthering the nation’s economic growth.
Watch how the NIHR Biomedical Research Centres are transforming patients' lives.
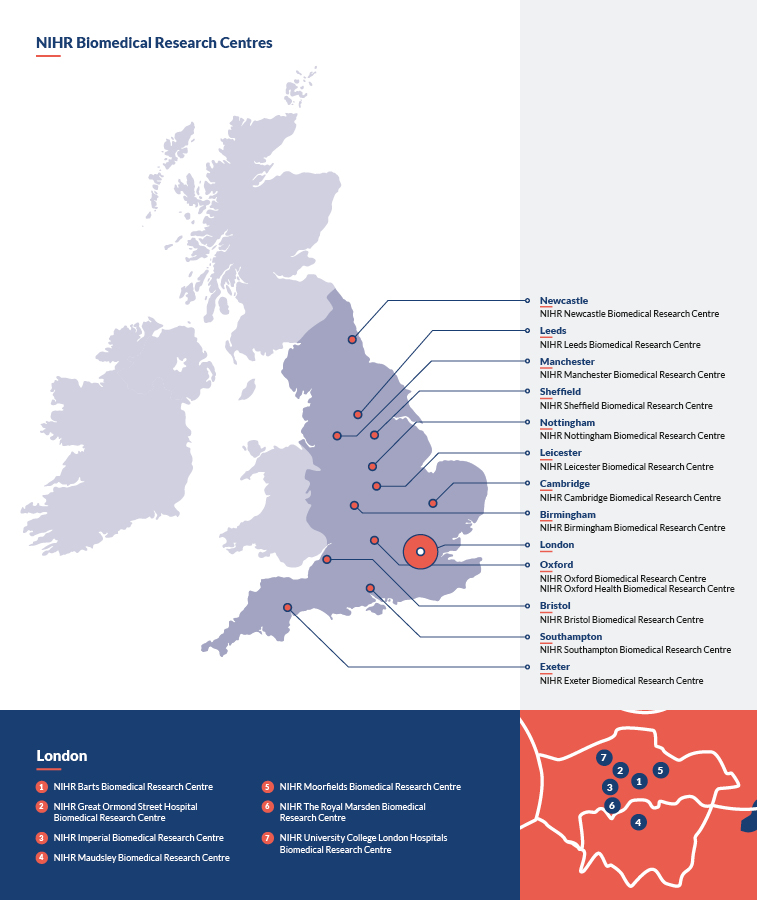
Where are the NIHR BRCs located?
BRCs are based within the NHS trusts hosting them and the collaborating universities. The 20 BRCs are:
- NIHR Barts Biomedical Research Centre
- NIHR Birmingham Biomedical Research Centre
- NIHR Bristol Biomedical Research Centre
- NIHR Cambridge Biomedical Research Centre
- NIHR Exeter Biomedical Research Centre
- NIHR Great Ormond Street Hospital Biomedical Research Centre
- NIHR Imperial Biomedical Research Centre
- NIHR Leeds Biomedical Research Centre
- NIHR Leicester Biomedical Research Centre
- NIHR Manchester Biomedical Research Centre
- NIHR Maudsley Biomedical Research Centre
- NIHR Moorfields Biomedical Research Centre
- NIHR Newcastle Biomedical Research Centre
- NIHR Nottingham Biomedical Research Centre
- NIHR Oxford Biomedical Research Centre
- NIHR Oxford Health Biomedical Research Centre
- NIHR The Royal Marsden Biomedical Research Centre
- NIHR Sheffield Biomedical Research Centre
- NIHR Southampton Biomedical Research Centre
- NIHR University College London Hospitals Biomedical Research Centre
What are the BRC themes?
Each BRC provides expertise across a broad range of health and disease areas. Their work is structured around a number of cross-cutting themes, including:
- Genomics
- Multimorbidity
- Data and digital health
- Imaging
- Immunology
- Precision medicine
- Rare diseases
The BRCs make use of their host organisation’s facilities, as well as other NIHR infrastructure such as NIHR Clinical Research Facilities, to conduct innovative research.
NIHR Translational Research Collaborations are hosted by BRCs, which collaborate with charity partners to develop and deliver early-phase translational research at scale. These collaborations convene UK-wide expertise in specific research areas addressing a gap or unmet need, serving as a platform to support emerging priorities in the research landscape and to co-ordinate efforts in early phase research.
Several BRCs support the work of the NIHR BioResource, which provides a national infrastructure for volunteers to be consented and recalled for research based on their genotype and phenotype, supporting human health research and its transformation into medical practice.
How do BRCs support research?
The BRCs receive sustained funding from NIHR to translate promising scientific breakthroughs and develop them into new treatments, diagnostics and medical technologies for the benefit of patients, the public and the health and care system. BRCs support research by:
- creating an environment where scientific endeavour can thrive, attracting the foremost talent and producing world-class outputs
- supporting a critical mass of people and infrastructure focused on biomedical innovation and early translational and experimental medicine research
- supporting capacity building through Early Career Fellowships and training/development opportunities
- delivering NIHR-funded research, while working with other public funders, charities and the life sciences industry
- leveraging and attracting funding from external organisations to undertake experimental medicine and early translational research while forming key strategic partnerships
BRC support for the life sciences industry
BRCs are designed to accelerate the translation of preclinical studies from experimental to early-phase research. They are skilled at overcoming bottlenecks and moving breakthroughs along the research pipeline.
For the life sciences industry, BRCs can provide:
- collaboration between globally recognised academic researchers, specialised NHS clinicians, the life sciences industry and other BRCs
- world-class laboratory and research facilities
- bespoke specialised equipment, such as sleep clinics, PET scanners, and simulation suites
- access to large and diverse patient populations
- access to tissue samples and health data through the NIHR BioResource testbed for translational research
They will also provide project management support for every stage of a study, including:
- care pathway and health economic analysis to identify unmet clinical needs
- protocol design, including patient-and-public involvement from the first stage of protocol development
- oversight of early phase studies across all specialties
The life sciences industry and sponsors of commercial research should contact the NIHR industry team to discuss their requirements and explore which facility would be best suited to support them.
Visit our ‘Offer to the Life Sciences Industry’ page to discover the full range of support available to commercial research sponsors developing and delivering research in the UK.
