Introduction
What is the Central Portfolio Management System (CPMS)?
The Central Portfolio Management System (CPMS) is a cloud-based system that holds the NIHR Clinical Research Network (CRN) Portfolio, as well as the network portfolios of Northern Ireland, Scotland and Wales.
CPMS is used by the CRN to support study management from set-up to completion. It is used by non-commercial study teams and the life-sciences industry to submit CRN service requests, and is the portal through which the interactive Costing Tool and online SoECAT can be accessed. Recruitment activity uploaded to CPMS, and other study data stored in CPMS, are used to support performance management of studies so that Portfolio studies achieve recruitment and time targets.
Types of studies on CPMS
Studies included on the NIHR Clinical Research Network (CRN) Portfolio and managed in CPMS must meet the eligibility criteria outlined by the Department of Health and Social Care. Further details of this and the requirements to access support and be included on the Portfolios of Northern Ireland, Scotland and Wales can be found on the NIHR website. Studies managed in CPMS include both non-commercial studies and commercial contract research.
Who uses CPMS and for what activities?
Company representatives (Commercial studies)
- Company representatives are able to access our free-of-charge feasibility services through the online submission platform in CPMS.
- Company representatives can also view the study records for the studies they have submitted to us.
National Portfolio Manager (NPM)
- These users are based at the Research Delivery Network Coordinating Centre (RDNCC) and equivalent within the devolved administrations.
- This role allows overall management of study records with complete editing and approval rights for all study record data.
- NPMs with additional funder viewer/manager permissions can view/edit restricted data in CPMS related to the eligibility of individual funding streams.
Local Portfolio Manager (LPM)
- These users are based at Local Clinical Research Networks (LCRNs).
- Local Portfolio Manager access allows some edit rights of study records to support the provision of study information and general troubleshooting. Some changes may need NPM approval.
- For Commercial studies, Local Portfolio Managers are able to provide site specific responses to commercial feasibility requests in CPMS.
AcoRD Specialist
- This is a new role added in CPMS Release 5 AcoRD Specialists may be based within an LCRN, or R&D office.
- AcoRD Specialists can view all SoECATs across the UK and provide authorisation on request
Study Resource Reviewer
- The Study Resource Reviewer is based in an LCRN or devolved admin
- Reviewers can only work on studies they are assigned to by an LPM
- Reviewers review all submitted Commercial Study Resource Review submissions and mark these submissions as ‘complete’ in CPMS
Non-commercial study teams
- These users are based within study teams. There are four study specific roles that provide access to specific study records associated with a user. Access to service submissions is role specific:
- Chief investigator: Can view/edit all submissions for studies they are named CI on (subject to submission status) and edit the study record*
- Study representative: Can view/edit SoECATs for studies they are associated with (subject to submission status) and edit the study record*
- Study coordinator: Can view/edit CRN Portfolio applications for studies they are associated with (subject to submission status) and edit the study record*
- Research activity coordinator: Can upload research activity data and edit the study record*
* Some changes may need approval by a National Portfolio Manager.
- Non-commercial study teams can also apply for ISRCTN registration via CPMS.
User guidance
How to log into CPMS
NIHR email address holders and existing account holders
Users of CPMS who have an NIHR email address, and any user previously registered on the HRA’s new IRAS system, can login through the Identity Gateway at cpms.nihr.ac.uk. Simply enter your email address, password and click continue.
All NPMs and LPMs should already have CPMS accounts with the relevant permission set. If not, they should contact their local IT support team or the Study Support Service Helpdesk at: supportmystudy@nihr.ac.uk.
Please note: In order for the SSS Helpdesk to authorise roles, requests for NPM permissions must come from that person’s line manager; and requests for LPM permissions must come from their COO or a nominated deputy.
Non-NIHR email address holders
Users of CPMS who do not have an NIHR email address or an existing account can create one through the Identity Gateway at cpms.nihr.ac.uk by following the steps below.
NOTE: If you have previously signed up for an account on the HRA’s new IRAS system you do not need to re-register to access CPMS (or vice-versa); trying to re-register will generate an error message.
- Select ‘create account’ on the sign-in page.

- Enter your email address.
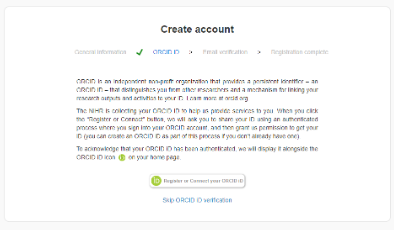
- You will then be directed to the ‘Create account’ page. Complete the details on the form and agree to the ‘Terms and Conditions’ before clicking the Next button.
- Select whether you want to register/connect your ORCID to your CPMS account. You can skip the ORCID verification if you prefer, it can be added at a later date.
- You will then be directed to a page asking you to validate your new CPMS account via a confirmation link sent to you via email.
- When your new account has been created and you have confirmed the link in your email, you can login to the Identity Gateway at cpms.nihr.ac.uk.
Logging in with Multi Factor Authentication (MFA)
When you log into CPMS for the first time you will be asked to input a One-Time Passcode. The Passcode will be sent automatically from identity@nihr.ac.uk to the email address associated with your CPMS account. Copy the six digit Passcode into the box on the login screen and click the Authenticate button. The Passcode is valid for 15 minutes.
Once you have logged into the system with your Passcode you will not need to do so again for CPMS, or any other system connected to the NIHR's Identity Gateway, for another 30 days, providing that your account is accessed from the same browser and device. After 30 days, or if you clear the cookies in your browser, you will be sent a new Passcode which will be good for a new 30 day period.
The Home page and navigation menu
Once logged in all users will arrive at the CPMS homepage. The options available in the side menu depend on the user's role and the permissions they hold. The options available can be viewed by clicking on the three lines in the top left-hand corner to expand the menu. 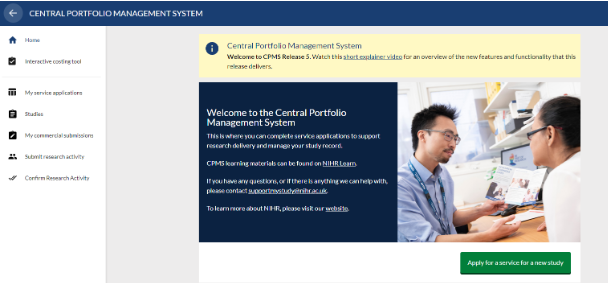
Applying for services
Company representatives and non-commercial study teams
The system will guide you through a series of screens to create an application for a new study.
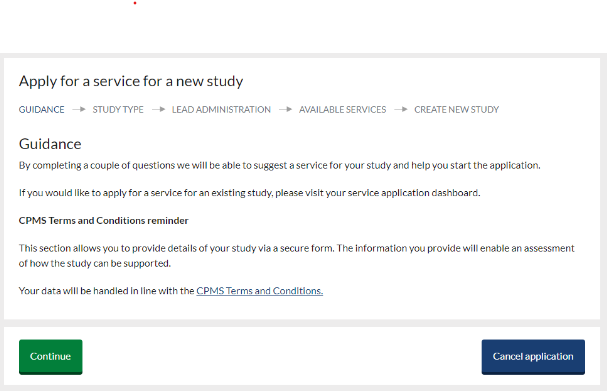
- To get started click the green ‘Apply for a service for a new study’ button on the home page. New services can be added to an existing study via your submission dashboard:
- My commercial submissions’ for company representatives
- ‘My service applications’ for non-commercial study teams
-
On the ‘Guidance’ screen read the information provided and if you are happy to proceed following the CPMS Terms and Conditions, select ‘Continue’.
-
On the ‘Study type’ screen select ‘Commercial’ or Non-Commercial’ and click ‘continue’. If you are not sure whether your study is a non-commercial or commercial study, contact us at supportmystudy@nihr.ac.uk for assistance before continuing.
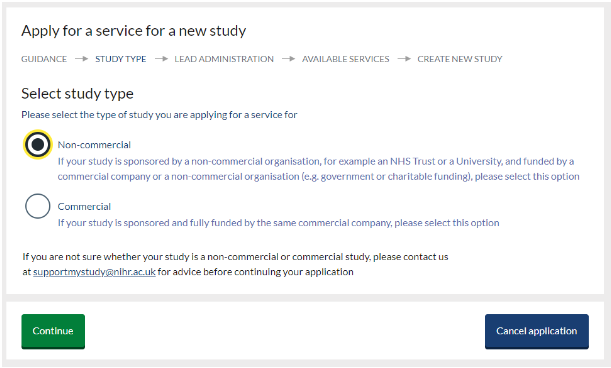
Commercial representatives
- Selecting ‘Commercial’ will direct you to the familiar commercial submission area of CPMS. From here you can apply for the services you require. Additional service specific guidance can be found on NIHR Learn.
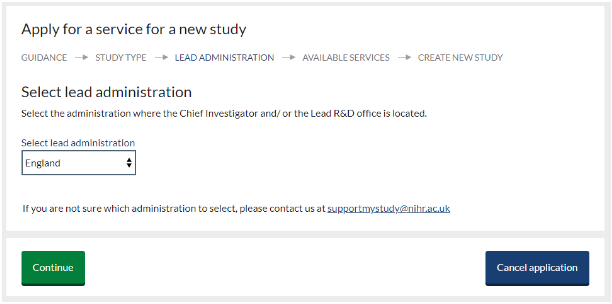
- On the ‘Available services’ screen select the service you require from the available options then click ‘continue’. You must ensure your study meets the requirement to use the selected service.
- Schedule of Events Cost Attribution Template (SoECAT)- UK wide
- Non-commercial Portfolio Application (England only, see NIHR website)
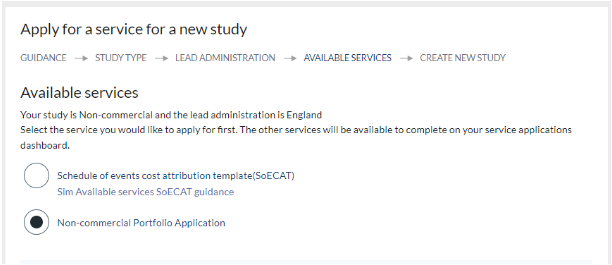
- You are now ready to enter the information required to create your new study record. All fields are mandatory. Please note the following:
- Enter the short and long title of your study. The information you enter will populate the study record and once submitted you will not be able to change it, so please ensure it is correct.
- All studies are required to obtain an IRAS ID. If you do not already have one, guidance on how to obtain an ID can be found within the IRAS system.
- You cannot make an application with an IRAS ID that is already associated with a study in CPMS. If this situation occurs, please contact us for assistance at: supportmystudy@nihr.ac.uk
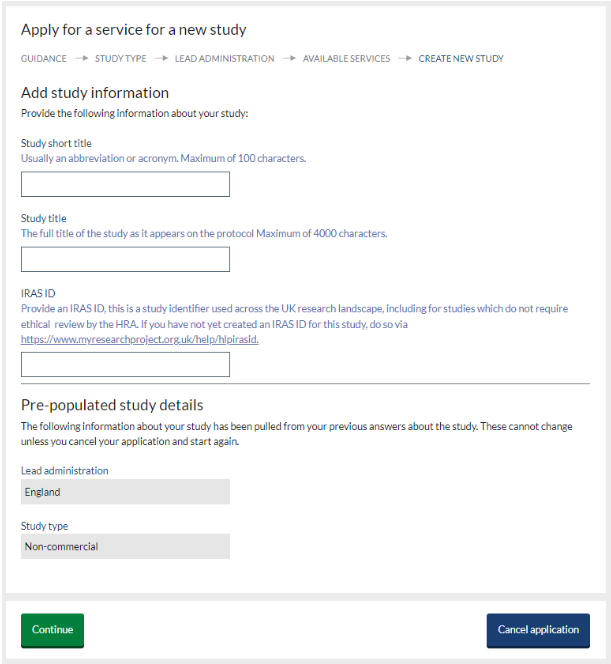
- Once you have populated all fields, click ‘continue’ to create your study record and access the requested form.
See the service specific guidance on NIHR Learn for further information on your required service.
If you cancel your application before this point, your information will not be saved.
Dashboards
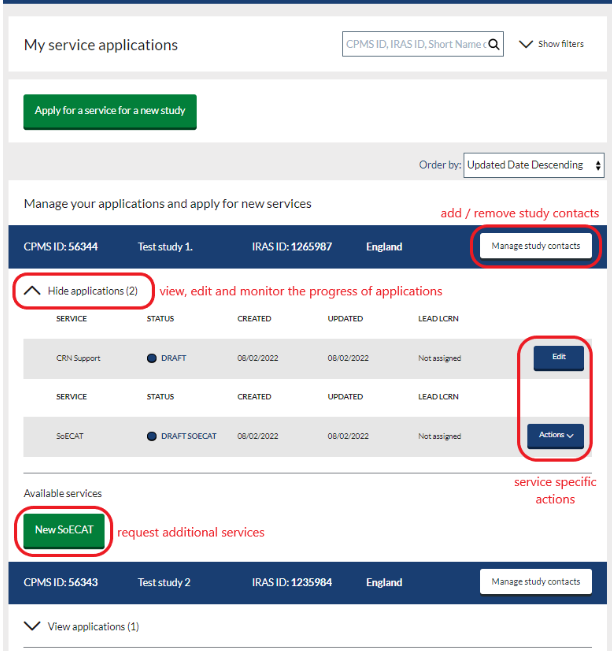
‘My service applications’ dashboard (non-commercial study teams)
- To view our applications through the integrated ‘My service applications’ dashboard, select this option from the menu on the homepage. This will direct you to the dashboard where all non-commercial applications associated with the user are listed.
-
Studies are listed with key details in the navy study header box. Details of associated service submissions can be viewed by clicking the down arrow to show/hide applications.
- The ‘My service applications’ dashboard provides information on each application and how it is progressing through the review process for each service. The current status of a study is shown on the dashboard beside a coloured dot. See service specific guidance on NIHR Learn for further details.
- From the ‘My service applications’ dashboard users can also undertake certain service specific actions associated with a submission and request additional services for their study by selecting the appropriate green service request option.
NOTE: Only one CRN Portfolio application is allowed per study, and this service is only available to English led studies.
- The list of applications can be searched, using the search box at the top of the page, and filtered by clicking ‘show filters’ and then selecting the required options. The list can also be sorted using the ‘order by’ drop down at the top right of the table. By default, the table is sorted with the newest applications at the top.
NOTE: Some sort options are only available once the dashboard has been filtered to show one specific service type.
- Study contacts can also be managed through the My service applications dashboard. See next section.
Commercial services dashboard
The ‘My commercial submissions’ dashboard is for company representatives to view their studies and associated submissions. Additional service specific guidance can be found on NIHR Learn.
NPM/LPM dashboards
In CPMS release 5, NPM and lPM users retain their individual service specific dashboards, accessed via the CPMS menu:
- IRAS submissions (NPM only)
- CRN Portfolio dashboard
- Commercial submissions dashboard
Managing study team contacts
Study contacts can be added and removed using the 'manage contacts' button in the study header row of the ‘My service applications' dashboard. This will enable them to collaborate on your submission and study record. When a contact is added, they will receive an email notification. Users must hold the correct study role to access submissions.
NOTE: If an NPM within the RDNCC adds a study representative or study coordinator they will also have to grant themselves the appropriate role via ‘manage contacts’ on the ‘My service applications' dashboard. 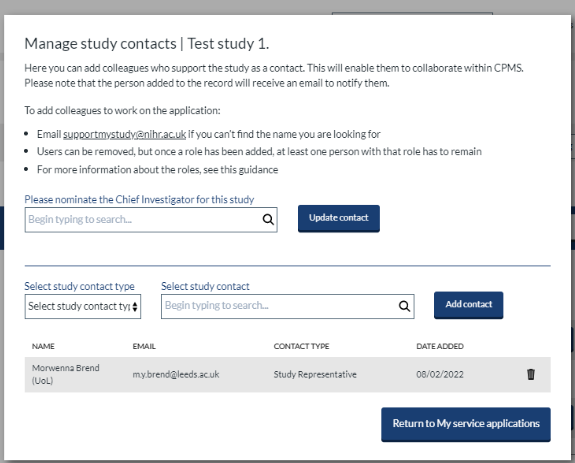
Managing study records
From the CPMS homepage, click to expand the menu and select ‘studies’. This will show you all the studies that are linked to your user name (company representatives or non-commercial study teams) or to your CRN staff role (NPM/LPM).
Currently public users of CPMS can access and edit any study record that they are associated with, as soon as it is created. However, they should be discouraged from doing so until the record has been made live, via usual business process. Editing the study record before this point will result in the record being locked.
In addition, applications for ISRCTN must only be made after the record has been checked and made live, otherwise the eligibility of the study for free registration will not be recognised by the system.
When you no longer require a CPMS account
If you leave your position or for other reasons no longer require a CPMS account, please ensure you submit a request to deactivate your CPMS account by emailing: crn.servicedesk@nihr.ac.uk.

